Phosphovimentin appeared as one of the proteins associated with biotinylated Mepiroxol plasma membrane-bound proteins in 5HT-stimulated platelets. SERT could be one of the other phosphovimentin-associated membrane proteins, but our co-IP data in 5HT-stimulated platelets also demonstrated an elevation in the association of SERTphosphovimentin in whole platelet. Therefore, we tested SERT-phosphovimentin association in 5HT-stimulated platelets. The effects of 5HT-stimulation on the amount of intracellular SERT mirrored those of the cell surface SERT. Previously, it has been shown that 5HT-stimulation phosphorylates vimentin on the Serine56 residue, but the vimentin S56A mutant is not phosphorylated by 5HT-stimulation. Therefore, to mechanistically determine how the vimentin-SERT association responds to 5HT for regulating the distribution of transporter molecules between plasma membrane and intracellular locations, the S56A mutant and the C-terminus truncated forms of SERT were studied in a CHO heterologous expression system. Obviously, not all aspects of 5HTbiology in platelets can be recapitulated in CHO cells, but the CHO model system allows for the analysis of the association between vimentin and the C-terminus truncated forms of SERT and the nonphosphorylated mutant form of vimentin S56A. To ascertain 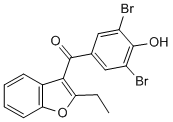 the optimal 5HT concentration required to stimulate CHO cells expressing hSERT, we measured the density of SERT proteins on the plasma membrane of CHO-hSERT cells and compared this finding to human platelet membranes using biotinylation. In this previous study, we tried to model the effect of plasma 5HT on platelet SERT in a heterologous expression system. Simply stated, an equal amount of biotinylated membrane proteins from CHO-SERT cells and platelets were resolved and analyzed by W/B using SERT-Ab. These calculations together with the dose response analysis of CHO cells to 5HT-stimulation which was already conducted in our previous studies showed that the expression of SERT on the plasma membrane of CHO-SERT cells was 59-fold higher than on the platelet membrane. This estimation indicated that the effect of plasma 5HT at a concentration of 1 nM on platelet SERT may Dexrazoxane hydrochloride correspond to exogenous 5HT at a concentration of,45 mM on CHO-SERT cells. The co-localization of the red vimentin and green YFP-SERT signals were captured in the overlaid images with YFP and Texas Red filter sets.
the optimal 5HT concentration required to stimulate CHO cells expressing hSERT, we measured the density of SERT proteins on the plasma membrane of CHO-hSERT cells and compared this finding to human platelet membranes using biotinylation. In this previous study, we tried to model the effect of plasma 5HT on platelet SERT in a heterologous expression system. Simply stated, an equal amount of biotinylated membrane proteins from CHO-SERT cells and platelets were resolved and analyzed by W/B using SERT-Ab. These calculations together with the dose response analysis of CHO cells to 5HT-stimulation which was already conducted in our previous studies showed that the expression of SERT on the plasma membrane of CHO-SERT cells was 59-fold higher than on the platelet membrane. This estimation indicated that the effect of plasma 5HT at a concentration of 1 nM on platelet SERT may Dexrazoxane hydrochloride correspond to exogenous 5HT at a concentration of,45 mM on CHO-SERT cells. The co-localization of the red vimentin and green YFP-SERT signals were captured in the overlaid images with YFP and Texas Red filter sets.
These studies also will advance our understanding of the specific processes by membrane via differential phosphorylation of SITPET sequence
Which facilitates the association of SERT with an intermediate filament, vimentin. Recent investigations indicate a system of Danshensu phosphorylation for SERT that incorporates two phases of phosphorylation. The first phase of phosphorylation is said to affect the serine residues, whereas the second phase involves the threonine residues. It is suggested that the first phase of phosphorylation causes the transporters to shut down, and the second phase of phosphorylation tags the proteins for internalization via the SERT recycling mechanism. According to the biphasic model, a S611D construct should shut down the uptake ability of the transporter while the DD construct should demonstrate a reduced or Butenafine hydrochloride eliminated 5HT uptake capacity due to its intracellular localization. Indeed, our data agree with the study by Jayanthi et al., who reported that S611D reduces transport capacity by,39%, whereas DD demonstrates an uptake capacity of,16%. On the basis of our findings, we hypothesize that the blunted activity of S611D may be due to additional serine residues that play a role in reducing the uptake capacity of SERT. A finding that was not consistent with the biphasic theory was the localization of S611D, which is mainly found at intracellular locations. In summary, in an endogenous platelet system and in heterologous expression systems, our studies demonstrate that vimentin associates with SERT. The last 20 amino acids from the C-terminus of SERT are required and are at least one of the binding-domain of vimentin. SERT 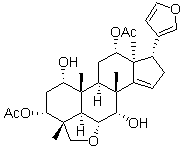 becomes a bridge between vimentin and the plasma membrane. At physiological plasma 5HT levels, vimentin-SERT association was found at intracellular locations and on the plasma membrane. However, when plasma 5HT level was higher than physiological level, their association was enhanced and the level of SERT on the plasma membrane was decreased. Therefore, we hypothesize that SERT utilizes the vimentin network during translocation from the plasma membrane. Furthermore, the 5HT-dependent phosphorylation of vimentin on the S56 residue accelerates the translocation of SERT on the 5HT-altered vimentin network. Future analysis of these mutants in stable transfection systems, as well as continued experiments with the phospho-mimicking mutants presented here, will further reveal the mechanism of action that governs transporter C-terminal phosphorylation.
becomes a bridge between vimentin and the plasma membrane. At physiological plasma 5HT levels, vimentin-SERT association was found at intracellular locations and on the plasma membrane. However, when plasma 5HT level was higher than physiological level, their association was enhanced and the level of SERT on the plasma membrane was decreased. Therefore, we hypothesize that SERT utilizes the vimentin network during translocation from the plasma membrane. Furthermore, the 5HT-dependent phosphorylation of vimentin on the S56 residue accelerates the translocation of SERT on the 5HT-altered vimentin network. Future analysis of these mutants in stable transfection systems, as well as continued experiments with the phospho-mimicking mutants presented here, will further reveal the mechanism of action that governs transporter C-terminal phosphorylation.
To capture a broad assessment of the molecular processes represented by the modulated genes
Finding clear evidence for disrupted coordination of mitochondrial gene expression led us to carryout a global analysis of skeletal Diperodon muscle phenotype using microarray technology to determine the extent of altered muscle phenotype. Informatic analysis yielded profound evidence for degeneration and loss of muscle specific genes. We found that regulation of apoptosis, proteosome function, ion homeostasis and kinase signalling were modulated and these appeared to us as rather predictable findings. Gene ontology analysis of the down regulated list indicated that there was a dramatic loss of unique muscle related gene expression demonstrating that the patient muscle tissue was undergoing a dedifferentiation process. Down regulated genes also indicated a loss of expression of numerous extracellular matrix gene ontology groups, which are known to be central to skeletal muscle remodelling and gain in physiological capacity. Thus, despite the lack of change in global protein synthesis, the types of protein being synthesised are most probably extremely different in the skeletal muscle of ICU patients. As we wish to intervene to prevent the loss of muscle tissue function, it is important to determine which biological pathways were regulating this shift in muscle phenotype. To examine this question, we took a number of approaches. Using a multiple array analysis strategy we utilised the extensive animal models of muscle wasting, inactivity and inflammation from the Goldberg laboratory. While it is implausible to directly assess muscle function in the ICU setting, 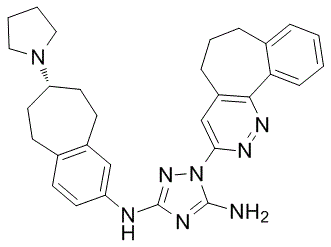 this comparative analysis allows us to contrast ICU patients with models of muscle atrophy, muscle inflammatory and muscle inactivity along the lines presented by Sacheck et al. It also facilitates discussion of the utility of such models and whether they accurately represent human muscle wasting disorders. In two recent publications, common genes up regulated or down regulated in animal models with muscle wasting have been identified. In the first study animal models for fasting, cancer cachexia, uremia and diabetes mellitus identified 120 unique genes involved in catabolism, and the authors named these ‘atrogens’. In a second paper these genes were Butenafine hydrochloride compared with two animal models of disuse induced muscle wasting, leading to 53 commonly changed genes.
this comparative analysis allows us to contrast ICU patients with models of muscle atrophy, muscle inflammatory and muscle inactivity along the lines presented by Sacheck et al. It also facilitates discussion of the utility of such models and whether they accurately represent human muscle wasting disorders. In two recent publications, common genes up regulated or down regulated in animal models with muscle wasting have been identified. In the first study animal models for fasting, cancer cachexia, uremia and diabetes mellitus identified 120 unique genes involved in catabolism, and the authors named these ‘atrogens’. In a second paper these genes were Butenafine hydrochloride compared with two animal models of disuse induced muscle wasting, leading to 53 commonly changed genes.
In MJD and arise through aberrant expansion of the polyglutamine encoding CAG repeat within the ataxin3
Following destabilization of native protein folding by expanded polyglutamine domains, 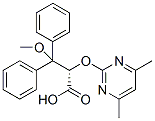 the aggregation of mutant huntingtin or ataxin-3 proceeds by nucleated growth polymerization into protein fibrils that structurally resemble amyloid fibrils and react with amyloid-specific histochemical dyes such as Congo Red or thioflavin. Analogous biophysical properties are observed for b-amyloid and a-synuclein fibrils associated with Alzheimer��s and Parkinson��s diseases, respectively. Amyloid fibrils are thought to be nucleated by monomers or globular oligomers of misfolded protein. In turn, fibrils may co-assemble into much larger insoluble protein aggregates that are resistant to proteolysis. While it is clear that protein misfolding can elicit cellular toxicity, whether fibrillar protein aggregates are Folinic acid calcium salt pentahydrate themselves toxic remains the subject of intense debate. On one hand, a growing body of evidence supports the “toxic soluble precursor” hypothesis in which end-stage protein fibrils are increasingly considered benign or even cytoprotective. In support of this hypothesis, small molecules that visibly stimulate inclusion-formation inside cells appear to be beneficial. However, contrasting studies have achieved Ginsenoside-F2 compelling cytoprotection by preventing fibrils and aggregates from forming at all. These competing hypotheses are not mutually exclusive given the growing diversity in “onpathway” and “off-pathway” protein folding conformations observed during amyloidogenesis. Conventional antibodies raised against amyloid conformations are known to cross-react with a wide variety of misfolded proteins, thereby illustrating that diverse amyloidogenic proteins share isomorphic features. However, these conformation-specific antibodies cannot be readily implemented inside living cells to investigate the conformational toxicity of intracellular amyloidogenic proteins such as huntingtin, a-synuclein, and ataxin-3 in situ. In an alternative approach, recombinant single-chain Fv antibodies, which preserve the binding specificities of monoclonal antibodies within the framework of a single small polypeptide, can be selected in vitro and expressed intracellularly as “intrabodies” to probe huntingtin and a-synuclein in living cells. By encoding the antigen-binding site of an immunoglobulin within the framework of a single nucleic acid coding sequence, scFvs are amenable .
the aggregation of mutant huntingtin or ataxin-3 proceeds by nucleated growth polymerization into protein fibrils that structurally resemble amyloid fibrils and react with amyloid-specific histochemical dyes such as Congo Red or thioflavin. Analogous biophysical properties are observed for b-amyloid and a-synuclein fibrils associated with Alzheimer��s and Parkinson��s diseases, respectively. Amyloid fibrils are thought to be nucleated by monomers or globular oligomers of misfolded protein. In turn, fibrils may co-assemble into much larger insoluble protein aggregates that are resistant to proteolysis. While it is clear that protein misfolding can elicit cellular toxicity, whether fibrillar protein aggregates are Folinic acid calcium salt pentahydrate themselves toxic remains the subject of intense debate. On one hand, a growing body of evidence supports the “toxic soluble precursor” hypothesis in which end-stage protein fibrils are increasingly considered benign or even cytoprotective. In support of this hypothesis, small molecules that visibly stimulate inclusion-formation inside cells appear to be beneficial. However, contrasting studies have achieved Ginsenoside-F2 compelling cytoprotection by preventing fibrils and aggregates from forming at all. These competing hypotheses are not mutually exclusive given the growing diversity in “onpathway” and “off-pathway” protein folding conformations observed during amyloidogenesis. Conventional antibodies raised against amyloid conformations are known to cross-react with a wide variety of misfolded proteins, thereby illustrating that diverse amyloidogenic proteins share isomorphic features. However, these conformation-specific antibodies cannot be readily implemented inside living cells to investigate the conformational toxicity of intracellular amyloidogenic proteins such as huntingtin, a-synuclein, and ataxin-3 in situ. In an alternative approach, recombinant single-chain Fv antibodies, which preserve the binding specificities of monoclonal antibodies within the framework of a single small polypeptide, can be selected in vitro and expressed intracellularly as “intrabodies” to probe huntingtin and a-synuclein in living cells. By encoding the antigen-binding site of an immunoglobulin within the framework of a single nucleic acid coding sequence, scFvs are amenable .
ERT resulted in a transporter that still retained approximately of its 5HT uptake rate
Further analysis of the difference between D20 and D14 truncations of the SERT C-terminus, which retained 0% compared to 90% transport rates, respectively, revealed the sequence Homatropine Bromide SITPET. Labetalol hydrochloride Within this region, there are 3 potential phosphorylation sites at S611, T613, and T616. Several studies have demonstrated that PKC modulators, such as bPMA, reduce SERT localization on the plasma membrane and blunt 5HT uptake capacity. Additionally, these studies also established an interaction of PP2A, a component of the protein phosphatase complex, with SERT. Based on these findings, we analyzed the effects of phosphorylation-mimicking amino acids on the 5HT uptake rate of SERT. Our results indicate that S611 may be a key site for phospho-regulation, since the single mutation of S611 to D caused a 61% decrease in 5HT uptake rate whereas the single mutation of S611 to A caused no reduction in 5HT uptake. T613 and T616 individually do not appear to be critical phospho-regulatory residues since neither mutation of T613 and T616 showed a similar level of 5HT uptake rate. However, it is possible that these sites work in conjunction with each other to modulate the function  of the transporter since our results indicate that the triple mutation DDD of 611, 613, and 616 retained only 5% of its 5HT uptake capacity as compared to control SERT. It is also important to note that the presence of such a large amount of negative charge on the end of the protein could cause alterations in protein folding or proteinprotein associations that are important for protein function, resulting in the observed blunting of transport capacity. Next, we analyzed the impact of four truncations of the SERT C-terminus on the trafficking and expression of SERT on the plasma membrane using biotinylation and IF assays. Our data indicate that depending on the amount of truncation from the Cterminus of SERT, there was altered localization of the transporter. Therefore, we carried out a biotinylation analysis on some of the phosphorylation-mimicking mutations in an effort to determine the plasma membrane localization of these mutants, i.e., whether the mutation arrests them intracellularly or whether the mutants can still traffic to the plasma membrane. The data indicate that 3 possible phosphorylation sites do contribute to the 5HT uptake rates of transporters via inhibiting their proceedings toward the plasma membrane.
of the transporter since our results indicate that the triple mutation DDD of 611, 613, and 616 retained only 5% of its 5HT uptake capacity as compared to control SERT. It is also important to note that the presence of such a large amount of negative charge on the end of the protein could cause alterations in protein folding or proteinprotein associations that are important for protein function, resulting in the observed blunting of transport capacity. Next, we analyzed the impact of four truncations of the SERT C-terminus on the trafficking and expression of SERT on the plasma membrane using biotinylation and IF assays. Our data indicate that depending on the amount of truncation from the Cterminus of SERT, there was altered localization of the transporter. Therefore, we carried out a biotinylation analysis on some of the phosphorylation-mimicking mutations in an effort to determine the plasma membrane localization of these mutants, i.e., whether the mutation arrests them intracellularly or whether the mutants can still traffic to the plasma membrane. The data indicate that 3 possible phosphorylation sites do contribute to the 5HT uptake rates of transporters via inhibiting their proceedings toward the plasma membrane.