In genomic DNA the fraction of double strand breaks repaired by HR varies in different cell types and is predominant in lower eukaryotes, whose smaller genome may allow homologous chromosomes to find each other more easily than those in higher eukaryotes. Similarly, HR may be favoured in the minichromosome due to the proximity of numerous replicating and daughter DNA molecules in replication compartments whose limited volume would facilitate finding a region of sequence homology in a neighbouring molecule. Linear oligomers of minichromosome DNA were not detected during repair, as also observed during repair of a 3 Mb double-minute chromosome and transfected plasmids, reflecting juxtaposition of the extremities of the broken DNA by Ku and the RMX complex; we propose that a further important factor is the crowded macromolecular Torin 1 environment in the nucleus because crowding strongly favours DNA circularisation and ligation 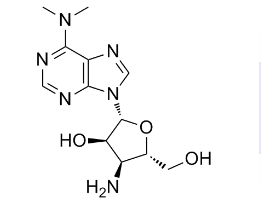 by ligases IIIb and IV-XRCC4 which participate in NHEJ. Kinetic models of strand break repair can be constructed with different degrees of complexity, but theory shows that the least complex model is preferable to provide concrete predictions. Our data were fitted well by using first-order kinetics, and we consider that this strategy was justified since other datasets for DNA repair have been fitted satisfactorily by first-order kinetics, which only deviate significantly from higherorder models after two half-times ; further, theoretical arguments show that “multiple processes may combine to produce kinetic behavior indistinguishable from firstorder and. are more likely to exist when reactions occur in a complex environment”. A number of conclusions which were not directly apparent from the experimental data LY2157299 illustrated the usefullness of modeling. First, when repair of double strand breaks was arrested, the single strand breaks in linear molecules were still repaired and circular molecules containing single strand breaks were converted to supercoiled molecules at close to the normal rate showing that the systems which repair single and double strand breaks operate independently, which has not been demonstrated previously as far as we are aware. Second, the calculated rate constants show that in an average linearised minichromosome the double strand break was repaired three to four times faster than all the single strand breaks, so that the ratelimiting step for complete repair of minichromosomes was the repair of single strand breaks. These repair rates cannot be compaired directly with those reported for genomic DNA where the methods used could not quantitate breaks directly, but comparisons can be made in terms of the half-time for repair which is independent of the radiation dose and of the length of the region considered. In the minichromosome the calculated half-time for repair of the double strand break in each molecule was,40 min, which is within the range of 20 to 110 min reported for genomic DNA. For repair of single strand breaks the half-time of,140 min for repairing 8 to 9 breaks per molecule was equivalent to an average of,16 min/break, which is within the range reported for genomic DNA. This minichromosome offers an simple experimental system for quantitative testing of potential inhibitors of repair of strand breaks, and since the sequence and structural features of its DNA and its transcription pattern have been studied extensively.
by ligases IIIb and IV-XRCC4 which participate in NHEJ. Kinetic models of strand break repair can be constructed with different degrees of complexity, but theory shows that the least complex model is preferable to provide concrete predictions. Our data were fitted well by using first-order kinetics, and we consider that this strategy was justified since other datasets for DNA repair have been fitted satisfactorily by first-order kinetics, which only deviate significantly from higherorder models after two half-times ; further, theoretical arguments show that “multiple processes may combine to produce kinetic behavior indistinguishable from firstorder and. are more likely to exist when reactions occur in a complex environment”. A number of conclusions which were not directly apparent from the experimental data LY2157299 illustrated the usefullness of modeling. First, when repair of double strand breaks was arrested, the single strand breaks in linear molecules were still repaired and circular molecules containing single strand breaks were converted to supercoiled molecules at close to the normal rate showing that the systems which repair single and double strand breaks operate independently, which has not been demonstrated previously as far as we are aware. Second, the calculated rate constants show that in an average linearised minichromosome the double strand break was repaired three to four times faster than all the single strand breaks, so that the ratelimiting step for complete repair of minichromosomes was the repair of single strand breaks. These repair rates cannot be compaired directly with those reported for genomic DNA where the methods used could not quantitate breaks directly, but comparisons can be made in terms of the half-time for repair which is independent of the radiation dose and of the length of the region considered. In the minichromosome the calculated half-time for repair of the double strand break in each molecule was,40 min, which is within the range of 20 to 110 min reported for genomic DNA. For repair of single strand breaks the half-time of,140 min for repairing 8 to 9 breaks per molecule was equivalent to an average of,16 min/break, which is within the range reported for genomic DNA. This minichromosome offers an simple experimental system for quantitative testing of potential inhibitors of repair of strand breaks, and since the sequence and structural features of its DNA and its transcription pattern have been studied extensively.
Monthly Archives: August 2019
Provides a good model for examining other facets of DNA breakage and repair
For example mapping strand breaks and comparing WZ4002 repair in transcribed and nontranscribed regions. Such studies may be relevant to the repair of DNA in genomic chromatin in view of the topological similarity of the minichromosome to chromatin loops and its position in regions of lower chromatin density within the nucleus where double strand breaks in genomic DNA and sites of their repair are predominantly localised. Prostate cancers usually present as androgen-dependent tumors, susceptible to growth arrest/apoptosis induced by androgen ablation therapy. Although initially effective, androgen ablation frequently leads to the PF-4217903 development of castration-resistant prostate cancer, which is generally also resistant to other available treatments. As such, castration resistance commonly marks the end stage form of prostate cancer and is the major obstacle in disease management. Development of castration-resistant prostate cancer is characteristically associated with marked increases in resistance to apoptosis, a major death pathway for drug action. Apoptosis resistance resulting from up-regulation of antiapoptotic genes and their products 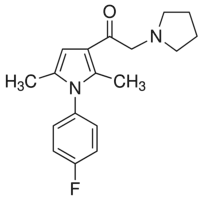 is thought to be a key contributor in the development of castration resistance, as well as general resistance to anti-cancer treatments. Elucidating the role of anti-apoptotic genes/proteins in the progression of prostate cancer is therefore likely to lead to improvements in the treatment of refractory disease. The Inhibitors of Apoptosis Protein family has been reported to play a role in apoptosis resistance in a variety of cancer cell lines and is characterized by the presence in the proteins of one to three copies of a Baculoviral IAP Repeat domain. The IAPs have been demonstrated to bind to and inhibit a variety of pro-apoptotic factors, thereby effectively suppressing apoptosis induced by a wide range of effectors, including chemotherapeutics and irradiation. The BIR domain is essential for interaction of the IAPs with pro-apoptotic factors, including caspases. The caspases are a family of cysteineaspartic acid-specific proteases, present in a pro-form which, once activated via cleavage, is responsible for degradation of death substrates such as poly-ADP-ribose polymerase thereby triggering apoptosis. Cleaved caspase-3 and cleaved PARP can be readily detected by Western blot analysis and are commonly used as markers for apoptosis. Apoptosis is often associated with autophagy, a process involving lysosomal degradation of a cell��s own components. It involves packaging of proteins and organelles within autophagosomes, followed by fusion with lysosomes leading to degradation of the proteins and organelles. The role of autophagy in the development of cancer and its treatment is complex, since there is evidence that autophagy can promote and suppress cancer growth. Inhibition of autophagy by disruption of essential autophagy genes has been shown to promote tumorigenesis and hence autophagy can have a tumor-suppressive effect. However, there is increasing evidence that autophagy can act as a survival mechanism for cancer cells in response to a wide range of stresses, including treatment with anti-cancer agents. To detect autophagic activity in cultured cells, Western blot detection of LC3B-II is often used. LC3B-II is specifically associated with autophagosomes and levels of LC3B-II have been demonstrated to correlate with the number of autophagosomes within cells. However, since LC3B-II is degraded upon autophagosome-lysosome fusion.
is thought to be a key contributor in the development of castration resistance, as well as general resistance to anti-cancer treatments. Elucidating the role of anti-apoptotic genes/proteins in the progression of prostate cancer is therefore likely to lead to improvements in the treatment of refractory disease. The Inhibitors of Apoptosis Protein family has been reported to play a role in apoptosis resistance in a variety of cancer cell lines and is characterized by the presence in the proteins of one to three copies of a Baculoviral IAP Repeat domain. The IAPs have been demonstrated to bind to and inhibit a variety of pro-apoptotic factors, thereby effectively suppressing apoptosis induced by a wide range of effectors, including chemotherapeutics and irradiation. The BIR domain is essential for interaction of the IAPs with pro-apoptotic factors, including caspases. The caspases are a family of cysteineaspartic acid-specific proteases, present in a pro-form which, once activated via cleavage, is responsible for degradation of death substrates such as poly-ADP-ribose polymerase thereby triggering apoptosis. Cleaved caspase-3 and cleaved PARP can be readily detected by Western blot analysis and are commonly used as markers for apoptosis. Apoptosis is often associated with autophagy, a process involving lysosomal degradation of a cell��s own components. It involves packaging of proteins and organelles within autophagosomes, followed by fusion with lysosomes leading to degradation of the proteins and organelles. The role of autophagy in the development of cancer and its treatment is complex, since there is evidence that autophagy can promote and suppress cancer growth. Inhibition of autophagy by disruption of essential autophagy genes has been shown to promote tumorigenesis and hence autophagy can have a tumor-suppressive effect. However, there is increasing evidence that autophagy can act as a survival mechanism for cancer cells in response to a wide range of stresses, including treatment with anti-cancer agents. To detect autophagic activity in cultured cells, Western blot detection of LC3B-II is often used. LC3B-II is specifically associated with autophagosomes and levels of LC3B-II have been demonstrated to correlate with the number of autophagosomes within cells. However, since LC3B-II is degraded upon autophagosome-lysosome fusion.
Leads to inhibition of autophagy and that BIRC6 may be a positive regulator of autophagy
The reduction in population growth induced by nontargeting siRNA is likely due to non-specific toxicity as reported by others ; importantly, it was not associated with an increase in INCB18424 molecular weight apoptosis marker expression. The results are consistent with reports of a critical role for BIRC6 in the survival of a variety of cancer cells. Cell cycle analysis showed that BIRC6 reduction did not result in significant change in cell cycle distribution, suggesting 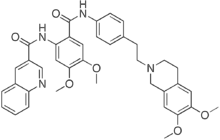 that the reduction in cell viability was attributable to apoptosis. In this study, a decrease in cell viability induced by BIRC6 reduction did not confine to cells expressing wild-type p53, contrary to previous reports suggesting that apoptosis resulting from BIRC6 knockdown in H460 cells and breast cancer cells requires functional p53. We showed that both wild type p53 and p53 null cells were sensitive to BIRC6 siRNA induced growth inhibition. This variation reflects that apoptosis induction by loss of BIRC6 may be facilitated by different mechanisms in different models. Further investigation is necessary to understand the underlying mechanism leading to apoptosis after BIRC6 reduction in p53 null cells and that will provide further insight in the possibility of targeting BIRC6 in cancer cells lacking functional p53. Elevated levels of BIRC6 have been linked to apoptosis resistance, for instance in the SNB-78 U0126 glioma cell line and over-expression of BIRC6 in human fibrosarcoma cells supports resistance to anti-cancer drugs and death receptor ligation. Furthermore, down-regulation of BIRC6 expression in SNB-78 cells was shown to sensitize the cells to apoptosis induced by cisplatin and camptothecin. It is therefore conceivable that the elevated expression of BIRC6 observed in castration-resistant prostate cancers may be responsible for the treatment resistance of refractory disease. The specific reduction of BIRC6 expression in LNCaP cells leading to a decrease in the expression of LC3B-II and Beclin-1 and decline in autophagosome accumulation, suggest that there is a novel role for BIRC6 in the regulation of autophagy. The reduced expression of LC3B-II indicates that loss of BIRC6 expression results in a lower number of autophagosomes. However, based on LC3B-II levels alone, it is not possible to determine whether the reduced number of autophagosomes is due to a decrease in autophagosome formation or to an increase in autophagosome degradation. To provide further insight into regulation of autophagy by BIRC6, BIRC6-depleted LNCaP cells were also examined for changes in the levels of Beclin-1. In mammalian cells enhanced expression of Beclin-1 has been shown to increase their autophagic response and the suppression of Beclin-1 has been shown to impair autophagy and sensitize cells to starvation-induced apoptosis. The reduced expression of Beclin-1 in the BIRC6-depleted LNCaP cells suggests that the lower numbers of autophagosomes in these cells is likely due to inhibition of autophagy initiation and autophagosome formation. In addition, in the LC3-GFP puncta formation assay where LNCaP cells were treated with chloroquine, a potent inhibitor of autophagosome degradation, BIRC6-depleted cells also showed less punctate structures than non-targeting controls. This evidence highlights the relationship between BIRC6 expression and autophagy initiation in particular. Taken together, these data demonstrate that loss of BIRC6 expression in LNCaP prostate cancer cells.
that the reduction in cell viability was attributable to apoptosis. In this study, a decrease in cell viability induced by BIRC6 reduction did not confine to cells expressing wild-type p53, contrary to previous reports suggesting that apoptosis resulting from BIRC6 knockdown in H460 cells and breast cancer cells requires functional p53. We showed that both wild type p53 and p53 null cells were sensitive to BIRC6 siRNA induced growth inhibition. This variation reflects that apoptosis induction by loss of BIRC6 may be facilitated by different mechanisms in different models. Further investigation is necessary to understand the underlying mechanism leading to apoptosis after BIRC6 reduction in p53 null cells and that will provide further insight in the possibility of targeting BIRC6 in cancer cells lacking functional p53. Elevated levels of BIRC6 have been linked to apoptosis resistance, for instance in the SNB-78 U0126 glioma cell line and over-expression of BIRC6 in human fibrosarcoma cells supports resistance to anti-cancer drugs and death receptor ligation. Furthermore, down-regulation of BIRC6 expression in SNB-78 cells was shown to sensitize the cells to apoptosis induced by cisplatin and camptothecin. It is therefore conceivable that the elevated expression of BIRC6 observed in castration-resistant prostate cancers may be responsible for the treatment resistance of refractory disease. The specific reduction of BIRC6 expression in LNCaP cells leading to a decrease in the expression of LC3B-II and Beclin-1 and decline in autophagosome accumulation, suggest that there is a novel role for BIRC6 in the regulation of autophagy. The reduced expression of LC3B-II indicates that loss of BIRC6 expression results in a lower number of autophagosomes. However, based on LC3B-II levels alone, it is not possible to determine whether the reduced number of autophagosomes is due to a decrease in autophagosome formation or to an increase in autophagosome degradation. To provide further insight into regulation of autophagy by BIRC6, BIRC6-depleted LNCaP cells were also examined for changes in the levels of Beclin-1. In mammalian cells enhanced expression of Beclin-1 has been shown to increase their autophagic response and the suppression of Beclin-1 has been shown to impair autophagy and sensitize cells to starvation-induced apoptosis. The reduced expression of Beclin-1 in the BIRC6-depleted LNCaP cells suggests that the lower numbers of autophagosomes in these cells is likely due to inhibition of autophagy initiation and autophagosome formation. In addition, in the LC3-GFP puncta formation assay where LNCaP cells were treated with chloroquine, a potent inhibitor of autophagosome degradation, BIRC6-depleted cells also showed less punctate structures than non-targeting controls. This evidence highlights the relationship between BIRC6 expression and autophagy initiation in particular. Taken together, these data demonstrate that loss of BIRC6 expression in LNCaP prostate cancer cells.