Indeed, recently it has been demonstrated that OCT-1 mediates cellular influx of imatinib, and that transporter activity correlates with efficacy. On the other hand, it has been shown that OCT-1 has less impact on cellular influx of dasatinib and nilotinib. Therefore, we believe that additional drug-transporter proteins contribute to TH-302 intracellular accumulation of TKIs. However, the data presented here is consistent with a model where intracellular accumulation and Life Science Reagents retention of TKIs in vivo also translates into significantly higher intracellular TKI concentrations as compared to the extracellular medium. It is conceivable that in the setting of high-dose pulse therapy this may then result in prolonged intracellular TKI exposure significantly exceeding plasma halflife of a given TKI. In conclusion, we show that dramatic intracellular TKI accumulation and retention result in prolonged target inhibition which appears to be the sole underlying molecular mechanism in HD-TKI pulse-exposure mediated induction of apoptosis in vitro. Moreover, the data illustrate that potent but transient kinase inhibition per se is not sufficient to irreversibly commit oncogene transformed cells to apoptosis. As we have observed intracellular TKI accumulation and retention in other oncogenic 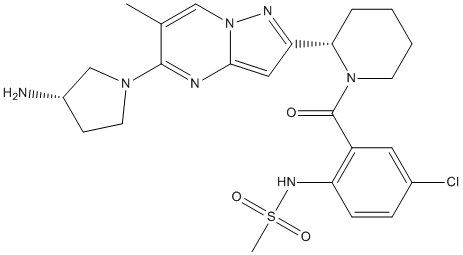 kinase models such as FLT3-ITD and JAK2V617F, the mechanism described here may indicate a general pharmacokinetic feature of TKIs. However, this point clearly requires further investigation. Based on our data presented here, monitoring both, plasma and intracellular drug levels of imatinib and dasatinib in vivo will provide pharmacokinetic data which may prove useful to optimize dosing schedules in upcoming clinical trials. We speculate that either the design of inhibitors that accumulate and are retained in target cells or, alternatively, co-administration of drugs which result in intracellular enrichment of specific TKIs may improve TKI therapy in the future. PLP is a reactive compound because the 49-aldehyde forms aldimines with a-amines of amino acids and other compounds containing amino groups, the e-amino group of lysine residues on non-B6 proteins, and thiazolidine adducts with sulfhydryl groups like cysteine. PL, which also contains the 49-aldehyde moiety is much less reactive than PLP, since its aldehyde group, in aqueous solution and at neutral pH, exists mostly in the hydrated form. This reactivity of PLP poses two problems for the cell. First, to keep the cellular level of free PLP low so that it does not react with other nucleophiles, especially non B6-enzymes, and second, to supply enough PLP for the dozens of newly synthesized apo-B6 enzymes to form catalytically active holo-B6 enzymes. Some have proposed that the level of PLP is regulated and kept low by being an effective feedback inhibitor of both PL kinase and PNP oxidase. We report here on properties of E. coli PL kinase showing that PLP serves as a slow tight binding inhibitor of the enzyme. The structure of PL kinase has been determined from several sources. In E. coli there are two PL kinases referred to as PL kinase1 and PL kinase2. The activity of PL kinase2 is very low and there is a question if its function in the cell is to convert PL to PLP or if it is the enzyme for another unknown reaction. We have determined the structure and properties of both E. coli enzymes. This study reports on the properties of only E. coli PL kinase1 and we refer to it as ePL kinase. During our kinetic measurements, we observed that the enzyme rapidly loses activity as it catalyzes the conversion of PL and ATP into PLP and ADP. The reason for this inhibition is the subject of this report.
kinase models such as FLT3-ITD and JAK2V617F, the mechanism described here may indicate a general pharmacokinetic feature of TKIs. However, this point clearly requires further investigation. Based on our data presented here, monitoring both, plasma and intracellular drug levels of imatinib and dasatinib in vivo will provide pharmacokinetic data which may prove useful to optimize dosing schedules in upcoming clinical trials. We speculate that either the design of inhibitors that accumulate and are retained in target cells or, alternatively, co-administration of drugs which result in intracellular enrichment of specific TKIs may improve TKI therapy in the future. PLP is a reactive compound because the 49-aldehyde forms aldimines with a-amines of amino acids and other compounds containing amino groups, the e-amino group of lysine residues on non-B6 proteins, and thiazolidine adducts with sulfhydryl groups like cysteine. PL, which also contains the 49-aldehyde moiety is much less reactive than PLP, since its aldehyde group, in aqueous solution and at neutral pH, exists mostly in the hydrated form. This reactivity of PLP poses two problems for the cell. First, to keep the cellular level of free PLP low so that it does not react with other nucleophiles, especially non B6-enzymes, and second, to supply enough PLP for the dozens of newly synthesized apo-B6 enzymes to form catalytically active holo-B6 enzymes. Some have proposed that the level of PLP is regulated and kept low by being an effective feedback inhibitor of both PL kinase and PNP oxidase. We report here on properties of E. coli PL kinase showing that PLP serves as a slow tight binding inhibitor of the enzyme. The structure of PL kinase has been determined from several sources. In E. coli there are two PL kinases referred to as PL kinase1 and PL kinase2. The activity of PL kinase2 is very low and there is a question if its function in the cell is to convert PL to PLP or if it is the enzyme for another unknown reaction. We have determined the structure and properties of both E. coli enzymes. This study reports on the properties of only E. coli PL kinase1 and we refer to it as ePL kinase. During our kinetic measurements, we observed that the enzyme rapidly loses activity as it catalyzes the conversion of PL and ATP into PLP and ADP. The reason for this inhibition is the subject of this report.
Monthly Archives: August 2019
Clinical management of these second most common type of skeletal malignancies after osteosarcoma has remained
It cannot be excluded that the interaction of PCI with the catalytically active light chain of EP is influenced by membrane anchoring of EP. However, the huge heavy chain lies in between the active center and the plasma membrane. This could hinder potential phospholipid-bridging of PCI and the light chain of EP. It is therefore not very likely that phospholipids involved in anchoring of EP could represent a bridge for bringing together PCI and EP. Several publications have shown that PCI mRNA is highly expressed in the pancreas, particularly 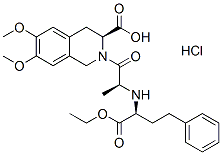 in the exocrine part. We could show by Western blotting that PCI protein is present in human pancreas lysate. However, we were not able to show its presence in the exocrine part by immunohistochemistry on paraffin-embedded tissue sections. Activation of SJN 2511 trypsinogen is a crucial step in the pathogenesis of necrotizing pancreatitis. So far, it is not fully understood how trypsinogen is activated prematurely within the pancreas. Some authors believe that this activation might origin from reflux of EPcontaining duodenal fluid into the pancreatic duct. Though, this theory remains controversial and it is not clear if duodenopancreatic reflux occurs in vivo and, if it does, whether it is really able to damage the pancreas profoundly. However, if duodenopancreatic reflux occurs under some circumstances, inhibition of EP by PCI might have a protective effect. In addition, PCI could protect the pancreas from autodigestion by inhibiting trypsin and chymotrypsin. Polymorphisms of PSTI are associated with a higher incidence of pancreatitis, suggesting that this inhibitor may have a protective effect against this disease. Polymorphisms of PCI have been investigated with respect to male fertility. Further studies are needed to address the question if polymorphisms of PCI might also be associated with pancreatitis. PCI has also been identified in the epidermis, with increasing amounts in its more superficial layers. Desquamation of keratinocytes is controlled by several proteases and their inhibitors. An imbalance between them can lead to inflammatory skin diseases such as psoriasis. Since both, EP and PCI, are expressed in the upper epidermis, the interaction of EP and PCI might also be involved in the regulation of epidermal differentiation. The SI value of the PCI/EP-interaction is rather high, especially in the presence of heparin, suggesting that a significant amount of PCI is cleaved/inactivated by EP. The biological consequences of the PCI/EP-interaction may depend on the local : ratio. At a high : ratio, the inhibition of EP would be nearly complete leading to impaired activation of trypsinogen. Additionally, some active PCI would still be available to inhibit trypsin directly, further reducing its activity. At a low : ratio, the available PCI would not be sufficient to completely inhibit EP. Remaining active EP would still be able to SP600125 JNK inhibitor activate trypsinogen and to cleave PCI. For the digestive system, this could mean that in the pancreatic duct, any possibly present EP could be efficiently inhibited by PCI. In the duodenum, where EP is abundantly present, PCI would have only a minor effect on EP activity and would not significantly interfere with the activation of digestive proteases. In the epidermis, where EP is mainly localized in the superficial layers, any EP present in more basal layers would be efficiently inhibited by excess PCI. In more superficial layers, EP would be able to activate trypsinogen in significant amounts, so it can contribute to the desquamation process. Chondrosarcomas constitute a heterogeneous group of neoplasms accounting for 20% of bone malignancies, that have in common the production of cartilage-like matrix by the tumor cells.
in the exocrine part. We could show by Western blotting that PCI protein is present in human pancreas lysate. However, we were not able to show its presence in the exocrine part by immunohistochemistry on paraffin-embedded tissue sections. Activation of SJN 2511 trypsinogen is a crucial step in the pathogenesis of necrotizing pancreatitis. So far, it is not fully understood how trypsinogen is activated prematurely within the pancreas. Some authors believe that this activation might origin from reflux of EPcontaining duodenal fluid into the pancreatic duct. Though, this theory remains controversial and it is not clear if duodenopancreatic reflux occurs in vivo and, if it does, whether it is really able to damage the pancreas profoundly. However, if duodenopancreatic reflux occurs under some circumstances, inhibition of EP by PCI might have a protective effect. In addition, PCI could protect the pancreas from autodigestion by inhibiting trypsin and chymotrypsin. Polymorphisms of PSTI are associated with a higher incidence of pancreatitis, suggesting that this inhibitor may have a protective effect against this disease. Polymorphisms of PCI have been investigated with respect to male fertility. Further studies are needed to address the question if polymorphisms of PCI might also be associated with pancreatitis. PCI has also been identified in the epidermis, with increasing amounts in its more superficial layers. Desquamation of keratinocytes is controlled by several proteases and their inhibitors. An imbalance between them can lead to inflammatory skin diseases such as psoriasis. Since both, EP and PCI, are expressed in the upper epidermis, the interaction of EP and PCI might also be involved in the regulation of epidermal differentiation. The SI value of the PCI/EP-interaction is rather high, especially in the presence of heparin, suggesting that a significant amount of PCI is cleaved/inactivated by EP. The biological consequences of the PCI/EP-interaction may depend on the local : ratio. At a high : ratio, the inhibition of EP would be nearly complete leading to impaired activation of trypsinogen. Additionally, some active PCI would still be available to inhibit trypsin directly, further reducing its activity. At a low : ratio, the available PCI would not be sufficient to completely inhibit EP. Remaining active EP would still be able to SP600125 JNK inhibitor activate trypsinogen and to cleave PCI. For the digestive system, this could mean that in the pancreatic duct, any possibly present EP could be efficiently inhibited by PCI. In the duodenum, where EP is abundantly present, PCI would have only a minor effect on EP activity and would not significantly interfere with the activation of digestive proteases. In the epidermis, where EP is mainly localized in the superficial layers, any EP present in more basal layers would be efficiently inhibited by excess PCI. In more superficial layers, EP would be able to activate trypsinogen in significant amounts, so it can contribute to the desquamation process. Chondrosarcomas constitute a heterogeneous group of neoplasms accounting for 20% of bone malignancies, that have in common the production of cartilage-like matrix by the tumor cells.
We recognized that MRLB-11055 had modest selectivity for signaling induced by EPO/JAK2 over signaling
Several mouse models of PV have been described that employ bone marrow transplantation of JAK2V617F to generate a phenotype that bears many of the hallmarks of disease. In all of these models, there is not only an expansion of erythrocytes, but also an expansion of the erythroid progenitor cells, which are their EPO receptor-expressing predecessors. PV patients are known to suffer from an increase in these cells, which appear as endogenous erythroid colonies in ex-vivo soft agar assays. In order to evaluate not only the effectiveness of an inhibitor but the optimal dose and NVP-BEZ235 schedule of that inhibitor, we reasoned that this progenitor population was the most likely candidate for the direct target tissue for the drug, and hence a key readout. Erythrocytes, as descendants of these cells, are indirectly targeted and with an inherent latency due to their lengthy half-life. Monitoring erythroid progenitors, however, is not readily achieved, and presents a challenge for assessment of optimal treatment time and holiday when developing a dosing schedule. For these reasons, we developed a JAK2V617F-Luciferase model system that allows realtime imaging of mutant expressing cell populations, which includes the erythroid progenitor population. A key consideration in the use of this model was the most appropriate stage in the progression of disease for introduction of the JAK2 inhibitor. We chose to administer the inhibitor to the mice at end of the 3rd week postBMT, at which time they were mildly polycythemic, with hematocrit levels actively rising. This state most  closely models the clinical condition of PV patients, who are not allowed to achieve plateau levels of Hct in normal care, and exist in a state of rising hematocrit between phlebotomy treatments. Under these conditions MRLB-11055 was observed to dramatically reduce the level of both erythroid progenitor cells and BLI in the spleen within a 3 day treatment period. While the exact mechanism of this reduction is not known, it is consistent with the rapid and robust induction of apoptosis in BaF3 cells dependent on JAK2V617F in vitro. When MRLB-11055 was removed, V617Fexpressing cells immediately began to Vorinostat abmole re-expand, consistent with the previous observation that the JAK2 mutation penetrates into the hematopoeitic stem cell population in these mice. Based on these observed kinetics of reduction and re-expansion, we were able to devise a multi-cycle intermittent dosing scheme aimed at normalizing progenitor populations. Application of this scheme not only prevented further rise of hematocrit in these mice, but actually decreased hematocrit to a level below the normal range. These decreases occurred even during the drug holiday period, clearly demonstrating that JAK inhibition need not be continuous to result in significant efficacy, and that hematocrit levels can be effectively managed by dosing schemes aimed at normalizing erythroid progenitor populations. JAK inhibitors have been described to have potent effects on lymphocyte subpopulations, prompting us to examine these lineages more closely. MRLB-11055 did indeed reduce T, B and NK cell fractions in the spleens of normal C57BL/6 mice when administered continuously at high doses. However, these reductions were significantly alleviated when MRLB-11055 was given intermittently according to the efficacious dosing schedule in the JAK2V617F-Luciferase mouse model. As immune function depends on the presence of these lymphoid cells, this data suggests that intermittent dosing could minimize immunodeficiencies induced by treatment with a JAK2 inhibitor.
closely models the clinical condition of PV patients, who are not allowed to achieve plateau levels of Hct in normal care, and exist in a state of rising hematocrit between phlebotomy treatments. Under these conditions MRLB-11055 was observed to dramatically reduce the level of both erythroid progenitor cells and BLI in the spleen within a 3 day treatment period. While the exact mechanism of this reduction is not known, it is consistent with the rapid and robust induction of apoptosis in BaF3 cells dependent on JAK2V617F in vitro. When MRLB-11055 was removed, V617Fexpressing cells immediately began to Vorinostat abmole re-expand, consistent with the previous observation that the JAK2 mutation penetrates into the hematopoeitic stem cell population in these mice. Based on these observed kinetics of reduction and re-expansion, we were able to devise a multi-cycle intermittent dosing scheme aimed at normalizing progenitor populations. Application of this scheme not only prevented further rise of hematocrit in these mice, but actually decreased hematocrit to a level below the normal range. These decreases occurred even during the drug holiday period, clearly demonstrating that JAK inhibition need not be continuous to result in significant efficacy, and that hematocrit levels can be effectively managed by dosing schemes aimed at normalizing erythroid progenitor populations. JAK inhibitors have been described to have potent effects on lymphocyte subpopulations, prompting us to examine these lineages more closely. MRLB-11055 did indeed reduce T, B and NK cell fractions in the spleens of normal C57BL/6 mice when administered continuously at high doses. However, these reductions were significantly alleviated when MRLB-11055 was given intermittently according to the efficacious dosing schedule in the JAK2V617F-Luciferase mouse model. As immune function depends on the presence of these lymphoid cells, this data suggests that intermittent dosing could minimize immunodeficiencies induced by treatment with a JAK2 inhibitor.
The interrogation of dosing the understanding of apoptosis in general regulation in particular
Furthermore, it can also help explain the mechanism behind bortezomib resistance in different tumors. Standard of care treatment for PV is phlebotomy supplemented with low-dose aspirin, and for intermediate to high risk patients follow-up hydroxyurea can be prescribed. While these treatment regimens have provided an excellent survival benefit, patients still suffer from reduced quality of life, owing to chronic fatigue, BMN673 pruritis and bone pain. Additionally patients are at significant risk of transformation to hematological malignancies such as AML. Treatment with JAK2 inhibitors can attenuate these symptoms, thus, there is genetic, cell based, and in vivo evidence to suggest a functional role for mutant JAK2 in the pathology of PV, and it is reasonable to predict that targeting the JAK2 protein could have therapeutic benefit in this patient population. In fact, the MPD community has been eagerly anticipating the PI-103 development of JAK inhibitors, and several are currently being tested in clinical trials. Given the broad role of JAK kinases in hematopoiesis, a key challenge will be not only the discovery of high quality targeted agents, but also effective methods of their use, as chronic, profound inhibition would likely be problematic. We have recently reported the discovery of a potent, orally active inhibitor of JAK2. Polycythemia vera is a disease involving biology for which there is a rich history of study. The discovery of the JAKV617F mutation shed light on the mechanism of disease origin and development. However,from the published literatureit appears that while JAK2 certainly plays an important role, other elements also likely contribute to the pathological evolution of PV. What is not knowniswhether inhibitionof the constitutively activated JAK2 mutant, signaling aberrantly downstream of the EPO receptor in erythroid and myeloid progenitor cells, will provide an effective improvement in the treatment of PV patients. To that end, several groups have developed pre-clinical models of PV disease, enabling the development of JAK2 inhibitors for evaluation in the clinic. A major challenge in development of any JAK2 inhibitor that is not selective for the V617F form of the enzyme is the expected mechanism-based toxicity, as JAK2 signaling is essential for many biological processes, within the hematopoeitic compartment and beyond. Chronic, high-level inhibition of JAK2 would almost certainly be intolerable, even if only considering the intended target tissues of the erythroid and myeloid lineage. Thus the dosing schedule of a JAK2 inhibitor is likely to be an important consideration in addition to the intrinsic properties of that inhibitor when considering its potential for successful clinical application. MRLB-11055 is a potent inhibitor of JAK2, however, similar to other described inhibitors of JAK2, it is not selective for JAK2V617F over JAK2WT. Despite this lack of selectivity at the enzyme level, cells that are dependent on JAK2V617F for growth are much more likely to commit to apoptosis in the presence of MRLB-11055 than their WT counterparts. This suggests a potential problem of adverse effects arising from chronic systemic JAK2 inhibition, and set the stage for exploring intermittent dosing in vivo. The pharmacokinetics of MRLB-11055 in mice was such that we were able to achieve sustained target inhibition each dosing cycle with once daily dosing, enabling efficacy studies. As MRLB-11055 was potent against JAK2WT, we were able to demonstrate efficacy in a model where PV-like symptoms, such as erythrocytosis and splenomegaly, could be rapidly generated by treatment of normal C57BL/6 mice with darbepoetin. While an important proof-of-concept for the inhibitor, this  model system is preventative.
model system is preventative.
A Poisson distribution of strand breaks was assumed DPP-IV inihbitors alone can reverse new-onset diabetes
The molecular events implicated in repair of CT99021 252917-06-9 strand breaks in DNA are becoming more clear, but an overall and quantitative picture of their repair in vivo which would contribute to understanding the systems biology of repair and the effects of inhibitors is not yet available. Current methods do not allow simultaneous and precise quantitation of repair of Perifosine single and double strand breaks. Repair of double strand breaks, which are believed to be the crucial lesions leading to cell death, is commonly assayed by restoration of the normal length of genomic DNA or restriction fragments using pulsed-field gel electrophoresis. Repair of single strand breaks, which may contribute to loss of viability by relaxing superhelical stress in genomic DNA loops and thus arresting transcription, cannot yet be quantitated specifically by methods with comparable precision. As a model system to approach this question we are studying the repair of strand breaks in vivo in a,170 kb circular minichromosome, the Epstein-Barr virus episome, which is maintained in the nuclei of Raji cells at 50�C100 copies localised at the periphery of interphase chromosomes. Two features of this minichromosome make it an attractive model for genomic chromatin: it can be considered as a defined region of chromatin in view of its canonical nucleosomal conformation and the well-studied sequence and properties of its DNA, and its closed circular topology and length resemble those of the constrained loops which genomic chromatin forms in vivo. After irradiating cells with 60Co c photons we assayed the repair of single strand breaks in the minichromosome by quantitating the loss of nuclease S1sensitive sites, and the repair of double strand breaks by PFGE assays of the reformation of supercoiled DNA from molecules which had been linearised. Circular molecules containing single strand breaks could not be quantitated directly, and instead their levels were calculated using a mathematical model developed to fit the experimental data. We exploited the possibility of quantitating repair in this system to examine the implication of particular enzymes, particularly topoisomerases I and II whose participation in repair has long been controversial, poly polymerase-1, Rad51, the catalytic subunit of DNA-protein kinase, and ATM kinase. New features of the repair of strand breaks in vivo and of their kinetics were revealed by mathematical modeling. The simultaneous repair of single and double strand breaks in a defined region of chromatin in vivo has not been studied previously using quantitative methods, to our knowledge. The methods used to detect strand breaks in earlier studies, filter elution or single-cell DNA electrophoresis, cannot provide absolute numbers of breaks and the reported rates were variable. We used two conditions to ensure that strand breaks were quantitated accurately: for PFGE, DNA was deproteinised at room temperature because extra strand breaks are created at higher temperatures, and hybridisation was carried out  in dried gels because the transfer of large DNA fragments onto membranes is not quantitative. In another study published while this manuscript was in preparation, a significant amount of minichromosome DNA remained in the sample well of PFGE gels and was interpreted as nicked circles, but here little or no DNA remained in the wells and nicked circular DNA migrated slowly into the gel, possibly reflecting methodological differences.
in dried gels because the transfer of large DNA fragments onto membranes is not quantitative. In another study published while this manuscript was in preparation, a significant amount of minichromosome DNA remained in the sample well of PFGE gels and was interpreted as nicked circles, but here little or no DNA remained in the wells and nicked circular DNA migrated slowly into the gel, possibly reflecting methodological differences.