SFA monotherapy did not suppress acute organ allograft rejection supporting the hypothesis that it does not represent a primary T cell inhibitor. Interestingly, in combination with CsA, SFA efficiently promoted long-term allograft survival. Furthermore, in a chronic allograft rejection model, addition of SFA to CsA-treated recipients markedly inhibited chronic rejection compared to animals receiving high dose CsA monotherapy, suggesting that SFA exerted unique immunobiological effects different from DAPT inhibition of calcineurin phosphatases. Current knowledge indicates that SFA represents a novel class of immunophilin-binding metabolite both with respect to chemical structure and functional activity. There is a paucity of knowledge about the immunobiological effects of SFA since each study focused on selected functions or selected aspects with professional antigen presenting cells being either directly or indirectly involved. Systematic studies investigating the effects of SFA are completely lacking. In this report we describe the results of the first systematic analysis of the immunobiological effects of the novel immunophilin-binding agent SFA on human monocyte-derived DC using a combination of genome-wide expression profiling with subsequent confirmation on the protein level and functional in vitro and in vivo assays. Results indicate that SFA represents a novel DC CX-4945 1009820-21-6 chemokine and migration inhibitor. Sanglifehrins represent novel immunosuppressive agents that have been reported to suppress key functions of DCs. We and others have reported that SFA inhibits bioactive IL-12p70 production, macropinocytosis as well as receptor-mediated endocytosis in human and murine DCs. Transplant experiments indicated that addition of SFA to CsA efficiently suppresses graft arteriosclerosis in comparison to CsA monotherapy suggesting that SFA may represent a novel class of immunophilin-binding agents. However, a disadvantage of previous studies is the fact that they have focused on selected molecules or selected functional aspects thereby restricting the possibility to discover novel mechanisms of action. Accordingly, the aim of the present study was to use a systematic genome-wide approach in order to reveal novel immunobiological effects of SFA on human DC. Secondly, identification of molecules being most specifically suppressed by SFA in comparison to the related molecule CsA may help to elucidate the mechanism of action. The results presented here indicate that SFA impairs DCmediated immunity in a so far unrecognized manner that is DC chemokine expression and migration. Importantly, SFA��s inhibitory effects can be demonstrated on two different functional levels such as direct chemokine expression inhibition and subsequent impaired attraction of CD4 helper T cells as wells as DC migration inhibition towards recombinant CCL19. Accordingly, we have found that SFA, in contrast to CsA, does not only inhibit mRNA and protein expression of a number of chemokines, including CCL5, CCL17 and CCL19 but additionally suppresses CD38 mRNA and DC surface expression. Thus, SFA��s effects on DC are unique in direct comparison to the related cyclophilin-binding immunosuppressant CsA. The latter results provide a rationale for the explanation of reduced migration of SFA-exposed moDCs against recombinant CCL19. CD38 has been reported to be required forthe migration of mature DC against recombinant CCL19. Furthermore, CD38 inhibition by SFA provides additional insight into recent reports demonstrating SFA��s capacity to abrogate bioactive IL-12 production in vitro and 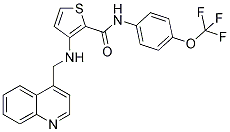 in vivo.
in vivo.
Monthly Archives: August 2019
Vascular regression and pruning followed by vascular maturation is required for the finalization of tissue reparative
The aim of this study was to probe if HDAC inhibition has any effect on human microvascular BMS-907351 pericytes with regards to proliferation, cell viability, migration and differentiation into profibrotic connective tissue cells in vitro. Furthermore, given the growing importance of the role of the pericyte in the regulation of the vasculature and the Talazoparib process of angiogenesis focus was directed towards potential modifications of expression patterns of mRNAs coding for proteins known to be involved in the angiogenic process in pericytes in response to HDAC inhibition. The effect of HDAC inhibition on pericyte proliferation was investigated. Inhibition of pericyte proliferation was seen already at a concentration of 1 mM VPA. Although the VPA analogue Penta which lacks HDAC inhibitory effects had a statistically significant effect on pericyte proliferation the effect did not approximate that observed in pericytes exposed to VPA suggesting that the anti-proliferative effect was to a large extent  due to VPAs ability to inhibit HDACs. However, a certain effect on pericyte proliferation due to the “off target effects” of VPA cannot be excluded. The notion that it is HDAC inhibition that is responsible for the effects seen on pericyte proliferation were further supported by experiments using TSA, another more potent HDAC inhibitor, which had similar anti-proliferative effects on pericytes when compared to VPA. The CFDA-SE incorporation experiments show that VPA, in a concentration dependent manner, limits the expansion of the pericyte population over the course of several cell divisions, and resulted in a higher percentage of pericytes retaining the dye. Previous studies addressing the potential role of HDAC inhibitors in blood vessel biology have focused on the endothelium. These in vitro results show that HDAC inhibition has an anti-proliferative effect also on pericytes and limits the expansion of the pericyte population over time. The present investigation shows that HDAC inhibition perturbs pericyte migration. When analyzing results from migration assays cell proliferation and viability must be taken into account in that a decrease in proliferation/viability may be misinterpreted as an inhibition of migration. In the present investigation no significant changes in the number of viable cells was seen after exposing pericytes to the concentrations of VPA used in the migration assay. Thus, cell death should not influence the observed results. At the 24-hour time point inhibitory effects on proliferation, but not migration, were seen in pericytes exposed to 1 mM VPA. VPAs inhibitory effect on migration was first significant in pericytes exposed to 3 mM VPA. Thus, a decrease in pericyte proliferation should not influence the observed results. The HDAC inhibitor TSA had a similar effect on pericyte migration. No effect on migration was observed when cells were exposed to the VPA analogue Penta suggesting that HDAC inhibition and not “off target effects” of VPA lead to the observed inhibition of pericyte migration. The results show that HDAC inhibition inhibits pericyte migration. The present investigation shows that VPA induces and upregulates expression of mRNAs coding for proteins involved in angiogenesis in pericytes in vitro. HDAC inhibition has been shown to inhibit angiogenesis in vivo and endothelial cell proliferation in vitro. No studies to our knowledge have addressed the role of pericytes in HDAC mediated effects on angiogenesis. Several pathological states in adults are initially characterized by an excessive and aberrant angiogenesis.
due to VPAs ability to inhibit HDACs. However, a certain effect on pericyte proliferation due to the “off target effects” of VPA cannot be excluded. The notion that it is HDAC inhibition that is responsible for the effects seen on pericyte proliferation were further supported by experiments using TSA, another more potent HDAC inhibitor, which had similar anti-proliferative effects on pericytes when compared to VPA. The CFDA-SE incorporation experiments show that VPA, in a concentration dependent manner, limits the expansion of the pericyte population over the course of several cell divisions, and resulted in a higher percentage of pericytes retaining the dye. Previous studies addressing the potential role of HDAC inhibitors in blood vessel biology have focused on the endothelium. These in vitro results show that HDAC inhibition has an anti-proliferative effect also on pericytes and limits the expansion of the pericyte population over time. The present investigation shows that HDAC inhibition perturbs pericyte migration. When analyzing results from migration assays cell proliferation and viability must be taken into account in that a decrease in proliferation/viability may be misinterpreted as an inhibition of migration. In the present investigation no significant changes in the number of viable cells was seen after exposing pericytes to the concentrations of VPA used in the migration assay. Thus, cell death should not influence the observed results. At the 24-hour time point inhibitory effects on proliferation, but not migration, were seen in pericytes exposed to 1 mM VPA. VPAs inhibitory effect on migration was first significant in pericytes exposed to 3 mM VPA. Thus, a decrease in pericyte proliferation should not influence the observed results. The HDAC inhibitor TSA had a similar effect on pericyte migration. No effect on migration was observed when cells were exposed to the VPA analogue Penta suggesting that HDAC inhibition and not “off target effects” of VPA lead to the observed inhibition of pericyte migration. The results show that HDAC inhibition inhibits pericyte migration. The present investigation shows that VPA induces and upregulates expression of mRNAs coding for proteins involved in angiogenesis in pericytes in vitro. HDAC inhibition has been shown to inhibit angiogenesis in vivo and endothelial cell proliferation in vitro. No studies to our knowledge have addressed the role of pericytes in HDAC mediated effects on angiogenesis. Several pathological states in adults are initially characterized by an excessive and aberrant angiogenesis.
The increased routine respiration in intact cells is due to an increase in the HK activity bound to mitochondria
In this pro-oxidant scenario, the ADP produced by high HK activity could control both membrane potential and ROS Afatinib generation through an ADP-recycling NSC 136476 mechanism similar to that proposed by Da-Silva et al. In connection with the mechanism of NaB activation of respiration, various interpretations, not necessarily mutually excluding, are possible. In one scenario, there could be a direct interaction of the HDACi with any of the mitochondrial components, similarly to what was described for HK. Conversely, NaB 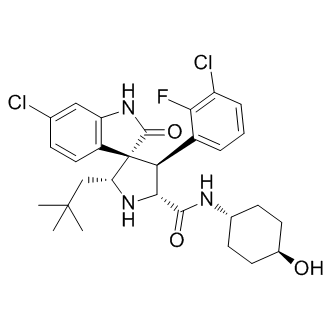 could act elsewhere and ultimately affect the respiratory parameters of the mitochondria. Perhaps the broadest consideration that must be taken into account is the possibility that most if not all the HDACis effects described in the present work could have been brought about by an action on a central factor that, in turn, would affect other pathways. One possibility is that HDACis are decreasing HIF-1a, and this effect decreases glycolysis maintained by HIF-1a. A key transcription factor that knowingly acts as the hub for many processes associated to the cell cycle and the energy metabolism itself. Thus, by preferentially targeting HIF-1a, NaB or TSA could indirectly affect hundreds of other genes. At any rate, investigation of the effect of HDACis on HIF1a regulation and on key actors of anaerobic and oxidative metabolism is currently under way. Although more evidence has yet to be procured, the set of results contained herein suggests that NaB and TSA could induce H460 cells to assume an altogether differentiated state. It is proposed that in this differentiated phenotype, cells actually shift towards more oxidative metabolism akin to untransformed cells. Ultimately, the enhanced oxidative metabolism would harm the H460 cells by way of excessive production of ROS that can cause cell death. In this context, the H460 cells treated with NaB could activate antioxidant defense mechanisms related to the increase in: HK activity bound to mitochondria, which increases ADP recycling; G6PDH activity, which increases PPP flux and NADPH supply; and the expression of Mfn 1, which, may reflect the initial stages of a process of mitochondrial fusion that can ultimately act as inhibitor of apoptosis. Indeed it has been shown that under oxidative stress mitochondria can rescue their membrane potential. Although the notion that NaB and TSA induced generation of ROS is a reasonable proposition, it must be mentioned that the topic of ROS and cancer cells is in itself quite controversial. There are data supporting the view that ROS are in fact essential requirements for the survival of tumor cells, even though the mechanism whereby ROS would support tumor growth remains elusive. Taken together the results presented here revealed a unique biochemical profile induced by NaB and TSA. The data essentially showed that alterations in the glycolytic flux triggered an enhancement of mitochondrial function that was not, however, paralleled by cell proliferation. Whilst further studies are paramount to unveil the mechanisms underlying the interplay between glycolysis and oxidative metabolism, the groundwork established in the present work already suggests that the stimulation of the oxidative metabolism in tumor cells may be an interesting strategy for chemotherapy. Ligand-based virtual screening, quantitative structureproperty and structure-activity relationships, and other concepts in computational medicinal chemistry are based on the similarity principle, which states that similar compounds generally exhibit similar properties.
could act elsewhere and ultimately affect the respiratory parameters of the mitochondria. Perhaps the broadest consideration that must be taken into account is the possibility that most if not all the HDACis effects described in the present work could have been brought about by an action on a central factor that, in turn, would affect other pathways. One possibility is that HDACis are decreasing HIF-1a, and this effect decreases glycolysis maintained by HIF-1a. A key transcription factor that knowingly acts as the hub for many processes associated to the cell cycle and the energy metabolism itself. Thus, by preferentially targeting HIF-1a, NaB or TSA could indirectly affect hundreds of other genes. At any rate, investigation of the effect of HDACis on HIF1a regulation and on key actors of anaerobic and oxidative metabolism is currently under way. Although more evidence has yet to be procured, the set of results contained herein suggests that NaB and TSA could induce H460 cells to assume an altogether differentiated state. It is proposed that in this differentiated phenotype, cells actually shift towards more oxidative metabolism akin to untransformed cells. Ultimately, the enhanced oxidative metabolism would harm the H460 cells by way of excessive production of ROS that can cause cell death. In this context, the H460 cells treated with NaB could activate antioxidant defense mechanisms related to the increase in: HK activity bound to mitochondria, which increases ADP recycling; G6PDH activity, which increases PPP flux and NADPH supply; and the expression of Mfn 1, which, may reflect the initial stages of a process of mitochondrial fusion that can ultimately act as inhibitor of apoptosis. Indeed it has been shown that under oxidative stress mitochondria can rescue their membrane potential. Although the notion that NaB and TSA induced generation of ROS is a reasonable proposition, it must be mentioned that the topic of ROS and cancer cells is in itself quite controversial. There are data supporting the view that ROS are in fact essential requirements for the survival of tumor cells, even though the mechanism whereby ROS would support tumor growth remains elusive. Taken together the results presented here revealed a unique biochemical profile induced by NaB and TSA. The data essentially showed that alterations in the glycolytic flux triggered an enhancement of mitochondrial function that was not, however, paralleled by cell proliferation. Whilst further studies are paramount to unveil the mechanisms underlying the interplay between glycolysis and oxidative metabolism, the groundwork established in the present work already suggests that the stimulation of the oxidative metabolism in tumor cells may be an interesting strategy for chemotherapy. Ligand-based virtual screening, quantitative structureproperty and structure-activity relationships, and other concepts in computational medicinal chemistry are based on the similarity principle, which states that similar compounds generally exhibit similar properties.
The CII transcriptional activator which is a key regulator of the lysis-lysogeny decision
This is a possibility since ATP-competitive kinase inhibitors bind within a related ATP-binding pocket. Using a TSA screen, we identified 12 small molecules that significantly shifted the thermal stability of DDK. Several of these functioned comparatively to PHA-767491 in the assay: the Rho kinase inhibitor, the PKR inhibitor, and the Chk1 inhibitor. These compounds fall into different structural classes indicating that significant chemical space is available for new DDK inhibitor development. Interestingly, UCN-01, also a Chk1 inhibitor related to staurosporine, was also identified in our screen and showed a high affinity for DDK. This raises the possibility that more potent and selective derivatives of staurosporine might be designed against DDK. It also raises the possibility that reported biological effects due to Chk1 inhibition may be enhanced by the ability of SB218078 and/or UCN-01 to also inhibit DDK. Rockout is a pyridine-substituted indole derivative and so is somewhat related to PHA-767491. However, the position of the pyridine moiety on the indole ring of Rockout is quite different from the geometry of PHA-767491. The PKR inhibitor also falls into a distinct structural class from either PHA767491 or XL413. It was noteworthy that the PKR inhibitor blocked the growth of HCC1954 breast cancer cells, induced apoptosis, and inhibited DDK-mediated Mcm2 phosphorylation nearly as well as the lead DDK inhibitor PHA-767491. RNA-dependent protein kinase is ubiquitously expressed protein that blocks protein synthesis in response to a number of stresses and impacts both neurodegenerative diseases and cancer through its ability to promote apoptosis. The particular PKR inhibitor we used inhibited PKR with an IC50 of 210 nM but inhibited DDK with an IC50 of,70 nM in vitro, and so should be classified as a dual PKR/DDK inhibitor. Whether the PKR inhibitor induced apoptosis in HCC1954 cells due to inhibiting DDK activity, PKR activity or both remains to be determined. In summary, our 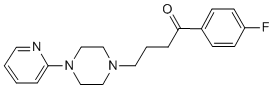 results highlight the cross-reactivity of several kinase inhibitors with DDK and also reveal an opportunity to develop more potent, biologically active DDK inhibitors for future evaluation. Proteolysis of key regulatory factors is an important control element of gene activity both in eukaryotic and prokaryotic cells. In bacteria degradation by ATP-dependent proteases, belonging to the AAA+ superfamily, participates in regulation of many developmental pathways: the heat shock response, starvation adaptation, DNA damage repair, capsular polysaccharide biosynthesis, sporulation and control of bacteriophage development. Specific adaptor proteins are known to modify the interaction of substrates with ATP-dependent proteases. However, there are only three known intracellular inhibitory polypeptides. The phage T4 PinA protein ASP1517 inhibits the Lon protease, and both the Bacillus species sporulation regulator SpoVM and the phage l CIII inhibit the FtsH protease. Both FtsH inhibitors, SpoVM and CIII, were predicted to form amphipathic a helices and are degraded by FtsH. The FtsH protease is the only essential ATP-dependent protease in E. coli.. It is a membrane-bound homohexamer enzyme made of three major domains: a trans-membrane domain, an ATPase domain and a protease domain. FtsH is complexed with HflKC forming an FtsH6-HflKC6 holoenzyme, which is present in the cell in less than 100 copies. FtsH degrades membrane proteins and a number of LY2157299 cytoplasmic proteins such as LpxC, s32, SsrA-tagged proteins and the bacteriophage lCII and CIII proteins. Degradation of LpxC by FtsH is required for Escherichia coli viability, as the levels of LpxC are essential for maintaining the balance in the synthesis of phospholipids and lipopolysaccarides. Bacteriophage l infection may activate either the lytic or the lysogenic developmental pathway. In l infection, physiological conditions as low temperature, starvation of the cells and high multiplicity of infection are known to favor lysogeny. A few phage functions are specifically required for the lysogenic response.
results highlight the cross-reactivity of several kinase inhibitors with DDK and also reveal an opportunity to develop more potent, biologically active DDK inhibitors for future evaluation. Proteolysis of key regulatory factors is an important control element of gene activity both in eukaryotic and prokaryotic cells. In bacteria degradation by ATP-dependent proteases, belonging to the AAA+ superfamily, participates in regulation of many developmental pathways: the heat shock response, starvation adaptation, DNA damage repair, capsular polysaccharide biosynthesis, sporulation and control of bacteriophage development. Specific adaptor proteins are known to modify the interaction of substrates with ATP-dependent proteases. However, there are only three known intracellular inhibitory polypeptides. The phage T4 PinA protein ASP1517 inhibits the Lon protease, and both the Bacillus species sporulation regulator SpoVM and the phage l CIII inhibit the FtsH protease. Both FtsH inhibitors, SpoVM and CIII, were predicted to form amphipathic a helices and are degraded by FtsH. The FtsH protease is the only essential ATP-dependent protease in E. coli.. It is a membrane-bound homohexamer enzyme made of three major domains: a trans-membrane domain, an ATPase domain and a protease domain. FtsH is complexed with HflKC forming an FtsH6-HflKC6 holoenzyme, which is present in the cell in less than 100 copies. FtsH degrades membrane proteins and a number of LY2157299 cytoplasmic proteins such as LpxC, s32, SsrA-tagged proteins and the bacteriophage lCII and CIII proteins. Degradation of LpxC by FtsH is required for Escherichia coli viability, as the levels of LpxC are essential for maintaining the balance in the synthesis of phospholipids and lipopolysaccarides. Bacteriophage l infection may activate either the lytic or the lysogenic developmental pathway. In l infection, physiological conditions as low temperature, starvation of the cells and high multiplicity of infection are known to favor lysogeny. A few phage functions are specifically required for the lysogenic response.
This synergy occurs both in the absence as well as the presence of stroma or stromal-derived cytokines
Resistance to TKIs in leukemia patients presents a significant clinical challenge. As small numbers of leukemia cells have been observed to persist in the bone marrow of TKI-treated patients, despite rapid and ALK5 Inhibitor II ALK inhibitor dramatic clearance of peripheral blood blasts, there is growing interest in determining the role of the bone marrow microenvironment in the long-term survival of leukemic stem cells. Indeed, the number of existing leukemic stem cells that exhibit high survival ability on bone marrow stromal layers has proven to be a significant prognostic indicator. Of relevance, we have found that media conditioned by human HS-5 stromal cells, as well as a cocktail of cytokines secreted in high concentrations by HS-5 stroma, were able to partially protect TKItreated chronic myeloid leukemia cells and AML cells. A subset of AML cells expresses a mutated form of the class III receptor tyrosine kinase FLT3, which has inspired the development of a number of small molecule inhibitors of mutant FLT3. However, FLT3 inhibitors tested thus far, including PKC412, which is in late stage clinical trials, and the highly potent and selective FLT3 inhibitor, AC220, which is in early phase clinical trials, generally at best induce partial and transient clinical responses in patients when used alone. In addition, we have found that bone marrow-derived stroma diminishes the activity of both PKC412 and AC220. There is thus a need for identification and development of novel therapies that can be effectively combined with TKIs to delay or suppress leukemia progression, override stroma-associated drug resistance, and increase patient survival. We have recently identified the multi-targeted kinase inhibitor, dasatinib, and dasatinib-like compounds as being able to potentiate the activity of TKIs PKC412 and AC220 against mutant FLT3-expressing cells cultured in the presence of cytoprotective and cytokine-abundant stromal-conditioned media by performing a combinatorial 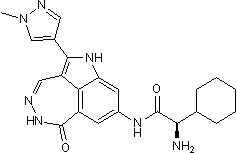 drug screen using the KIN001 library. Our study also highlighted the potential of Jak inhibitors to synergize with PKC412 and AC220 as well as enhance their apoptotic activity against mutant FLT3-expressing cells cultured in the presence of SCM. While the significance of stromal-derived growth factors in viability enhancement and cytoprotection of leukemic stem cells cannot be denied, not all hematologic malignancies can be rescued from programmed cell death by secreted cytokines in the absence of direct communication with the stromal cells themselves. As examples, protection of AML cells and B-lineage ALL cells from spontaneous and/or drug-induced apoptosis was observed to depend on direct bone marrow fibroblast cell:leukemic cell interaction. Similarly, protection of CLL cells from apoptosis depends on adherence of these cells to bone marrow stromal layers, and adhesion between bone marrow stroma and myeloma cells is necessary for protection of these cells from drug-induced apoptosis. Thus, the direct interaction between stromal cells and leukemic cells is important to fully GSK212 understand the mechanisms driving stromal-mediated chemoresistance, as well as for identification of integral signaling molecules as potential therapeutic targets for overriding drug resistance. To address this, we used an adherent stroma-based co-culture system, as opposed to the SCM-based system used previously, as the basis for a combinatorial drug screen designed to identify novel kinase inhibitors able to potentiate the apoptosis-inducing effects of PKC412 against adherent stroma-protected mutant FLT3positive cells. In parallel to the KIN001 kinase inhibitor library, we also screened the LINCS kinase inhibitor library, which is composed of inhibitors characterized as being relatively potent and selective toward a limited range of kinase targets. Here, we identified selective Akt inhibitors, such as MK2206, as able to effectively combine with FLT3 inhibitors, including PKC412 and AC220, against mutant FLT3-expressing cell lines or primary AML cells cultured in a cytoprotective stromal environment.
drug screen using the KIN001 library. Our study also highlighted the potential of Jak inhibitors to synergize with PKC412 and AC220 as well as enhance their apoptotic activity against mutant FLT3-expressing cells cultured in the presence of SCM. While the significance of stromal-derived growth factors in viability enhancement and cytoprotection of leukemic stem cells cannot be denied, not all hematologic malignancies can be rescued from programmed cell death by secreted cytokines in the absence of direct communication with the stromal cells themselves. As examples, protection of AML cells and B-lineage ALL cells from spontaneous and/or drug-induced apoptosis was observed to depend on direct bone marrow fibroblast cell:leukemic cell interaction. Similarly, protection of CLL cells from apoptosis depends on adherence of these cells to bone marrow stromal layers, and adhesion between bone marrow stroma and myeloma cells is necessary for protection of these cells from drug-induced apoptosis. Thus, the direct interaction between stromal cells and leukemic cells is important to fully GSK212 understand the mechanisms driving stromal-mediated chemoresistance, as well as for identification of integral signaling molecules as potential therapeutic targets for overriding drug resistance. To address this, we used an adherent stroma-based co-culture system, as opposed to the SCM-based system used previously, as the basis for a combinatorial drug screen designed to identify novel kinase inhibitors able to potentiate the apoptosis-inducing effects of PKC412 against adherent stroma-protected mutant FLT3positive cells. In parallel to the KIN001 kinase inhibitor library, we also screened the LINCS kinase inhibitor library, which is composed of inhibitors characterized as being relatively potent and selective toward a limited range of kinase targets. Here, we identified selective Akt inhibitors, such as MK2206, as able to effectively combine with FLT3 inhibitors, including PKC412 and AC220, against mutant FLT3-expressing cell lines or primary AML cells cultured in a cytoprotective stromal environment.