Several mouse models of PV have been described that employ bone marrow transplantation of JAK2V617F to generate a phenotype that bears many of the hallmarks of disease. In all of these models, there is not only an expansion of erythrocytes, but also an expansion of the erythroid progenitor cells, which are their EPO receptor-expressing predecessors. PV patients are known to suffer from an increase in these cells, which appear as endogenous erythroid colonies in ex-vivo soft agar assays. In order to evaluate not only the effectiveness of an inhibitor but the optimal dose and NVP-BEZ235 schedule of that inhibitor, we reasoned that this progenitor population was the most likely candidate for the direct target tissue for the drug, and hence a key readout. Erythrocytes, as descendants of these cells, are indirectly targeted and with an inherent latency due to their lengthy half-life. Monitoring erythroid progenitors, however, is not readily achieved, and presents a challenge for assessment of optimal treatment time and holiday when developing a dosing schedule. For these reasons, we developed a JAK2V617F-Luciferase model system that allows realtime imaging of mutant expressing cell populations, which includes the erythroid progenitor population. A key consideration in the use of this model was the most appropriate stage in the progression of disease for introduction of the JAK2 inhibitor. We chose to administer the inhibitor to the mice at end of the 3rd week postBMT, at which time they were mildly polycythemic, with hematocrit levels actively rising. This state most 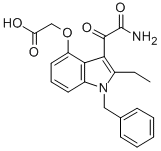 closely models the clinical condition of PV patients, who are not allowed to achieve plateau levels of Hct in normal care, and exist in a state of rising hematocrit between phlebotomy treatments. Under these conditions MRLB-11055 was observed to dramatically reduce the level of both erythroid progenitor cells and BLI in the spleen within a 3 day treatment period. While the exact mechanism of this reduction is not known, it is consistent with the rapid and robust induction of apoptosis in BaF3 cells dependent on JAK2V617F in vitro. When MRLB-11055 was removed, V617Fexpressing cells immediately began to Vorinostat abmole re-expand, consistent with the previous observation that the JAK2 mutation penetrates into the hematopoeitic stem cell population in these mice. Based on these observed kinetics of reduction and re-expansion, we were able to devise a multi-cycle intermittent dosing scheme aimed at normalizing progenitor populations. Application of this scheme not only prevented further rise of hematocrit in these mice, but actually decreased hematocrit to a level below the normal range. These decreases occurred even during the drug holiday period, clearly demonstrating that JAK inhibition need not be continuous to result in significant efficacy, and that hematocrit levels can be effectively managed by dosing schemes aimed at normalizing erythroid progenitor populations. JAK inhibitors have been described to have potent effects on lymphocyte subpopulations, prompting us to examine these lineages more closely. MRLB-11055 did indeed reduce T, B and NK cell fractions in the spleens of normal C57BL/6 mice when administered continuously at high doses. However, these reductions were significantly alleviated when MRLB-11055 was given intermittently according to the efficacious dosing schedule in the JAK2V617F-Luciferase mouse model. As immune function depends on the presence of these lymphoid cells, this data suggests that intermittent dosing could minimize immunodeficiencies induced by treatment with a JAK2 inhibitor.
closely models the clinical condition of PV patients, who are not allowed to achieve plateau levels of Hct in normal care, and exist in a state of rising hematocrit between phlebotomy treatments. Under these conditions MRLB-11055 was observed to dramatically reduce the level of both erythroid progenitor cells and BLI in the spleen within a 3 day treatment period. While the exact mechanism of this reduction is not known, it is consistent with the rapid and robust induction of apoptosis in BaF3 cells dependent on JAK2V617F in vitro. When MRLB-11055 was removed, V617Fexpressing cells immediately began to Vorinostat abmole re-expand, consistent with the previous observation that the JAK2 mutation penetrates into the hematopoeitic stem cell population in these mice. Based on these observed kinetics of reduction and re-expansion, we were able to devise a multi-cycle intermittent dosing scheme aimed at normalizing progenitor populations. Application of this scheme not only prevented further rise of hematocrit in these mice, but actually decreased hematocrit to a level below the normal range. These decreases occurred even during the drug holiday period, clearly demonstrating that JAK inhibition need not be continuous to result in significant efficacy, and that hematocrit levels can be effectively managed by dosing schemes aimed at normalizing erythroid progenitor populations. JAK inhibitors have been described to have potent effects on lymphocyte subpopulations, prompting us to examine these lineages more closely. MRLB-11055 did indeed reduce T, B and NK cell fractions in the spleens of normal C57BL/6 mice when administered continuously at high doses. However, these reductions were significantly alleviated when MRLB-11055 was given intermittently according to the efficacious dosing schedule in the JAK2V617F-Luciferase mouse model. As immune function depends on the presence of these lymphoid cells, this data suggests that intermittent dosing could minimize immunodeficiencies induced by treatment with a JAK2 inhibitor.
We recognized that MRLB-11055 had modest selectivity for signaling induced by EPO/JAK2 over signaling
Leave a reply