A further advantage is that the white fur of this albino strain makes in vivo imaging more sensitive and simpler to perform than in mice with black fur. This finding was not anticipated since MOPCs are generally considered to be poor models of MM bone disease, even though they readily form extramedullary plasmacytomas after local injection. It may be that Mechlorethamine hydrochloride MOPC315 has an increased tropism for bone marrow compared to other MOPC lines and bone marrow tropism may thereby vary Folinic acid calcium salt pentahydrate between different MOPC lines. The results further demonstrate that variants of the MOPC315 cell line can be obtained that more rapidly induces MMlike bone disease. Thus, a cell line selected for high tumor take by s.c. injection, MOPC315.4, caused paraplegia in all i.v.-injected mice within 65 days. After 9 cycles of i.v. injection of MOPC315.4 and recovery of cells from femurs of paraplegic mice, the MOPC315.BM cell line was obtained that following i.v. injection caused paraplegia in all mice within 35 days. It is unclear whether the consecutive s.c. and i.v. selection procedures resulted in either a gradual change of phenotype or in a selection of rare cells with more MM-like features, pre-existing in the parental plasmacytoma cell line. The fact that decreasing amounts of injected cells were needed with progressive cycles could indicate that the in vivo-selection might have enriched for a pre-existing variant. It is also unknown whether preferential growth in bone marrow was due to increased homing to the bone, or if cells simply grew better once they had settled in the bone marrow microenvironment or both. MOPC315.BM cells labeled with firefly luciferase could be followed by repeated bioluminescent imaging of i.v.-injected mice. Overall, the results were similar in normal BALB/c and T cell-deficient BALB/c nu/nu mice. However, sensitivity was clearly higher in furless BALB/c nu/nu mice, the nude strain being the recommended model for DLIT. Sensitivity was further increased by injection of a higher number of cells. Immediately after injection, cells were found primarily in the lung, but also in the spleen and the liver. However, and importantly, a minor fraction of cells were found in the tibiofemoral region already 1 h after injection. Early invasion of bone marrow is consistent with results obtained with i.v. injection of 51Cr-labeled 5T2MM cells. The spleen signal progressed with time, while lung and liver signals decreased. Affection of other organs was only infrequently detected. MM growth in spleen is consistent with extramedullary hematopoiesis in this organ, and is also found in the 5TMM models. It is generally believed that MM cells represent malignant counterparts of plasma cells that at the earlier B cell stage have been through a germinal center reaction. However, it is unclear where the neoplastic process initially takes place. One possibility is that MM originates from plasma cells malignantly  transformed within the bone marrow, and that MM cells later metastasize to other bones. Another possibility is that neoplastic cells originate in an extramedullary site, and then seed multiple bones where they are exposed to a microenvironment conducive to growth. Several pieces of evidence support the latter possibility. Firstly, MM has been associated with less differentiated clonogenic precursors found in blood. Secondly, extramedullary plasmacytomas in humans can metastasize to bone. Thirdly, ileocecal plasmacytoma in an aged gonadectomized mouse, as well as transplanted MOPC tumors, can metastasize to the bone marrow.
transformed within the bone marrow, and that MM cells later metastasize to other bones. Another possibility is that neoplastic cells originate in an extramedullary site, and then seed multiple bones where they are exposed to a microenvironment conducive to growth. Several pieces of evidence support the latter possibility. Firstly, MM has been associated with less differentiated clonogenic precursors found in blood. Secondly, extramedullary plasmacytomas in humans can metastasize to bone. Thirdly, ileocecal plasmacytoma in an aged gonadectomized mouse, as well as transplanted MOPC tumors, can metastasize to the bone marrow.
Monthly Archives: June 2019
An important aspect of the current model is that experiments can be perform have been established in immunodeficient
In particular, models have been generated where human MM cells grow in human fetal bone transplants in immunodeficient SCID mice. More recently three-dimensional bone-like scaffolds were coated with mouse or human bone marrow stromal cells and implanted under the skin of SCID mice. Subsequently, injection of purified primary myeloma cells into these scaffolds gave rise to tumor formation that could be followed by measuring myeloma protein concentration. Although these models allow experiments of human MM cells in vivo in mice, the models are demanding and not completely physiological. Mouse models where MM cells can be transferred Tulathromycin B between syngeneic mice are also available. However, mouse MM models do not necessarily accurately reflect human disease. MM-like disease arises spontaneously in aged C57BL/KaLwRij mice. The 5T2MM and the 5T33MM cell lines were established from such mice, and have been extensively used for studying homing mechanisms of MM cells to bone marrow, interaction of MM cells with the bone marrow environment, and evaluation of new therapies. Both models are characterized by MM cell infiltration restricted to bone marrow and spleen. The 5T2MM model, but not 5T33MM, is associated with an extensive osteolysis, seen on plain radiographs of femur and tibia. Finally, three different transgenic mice models have recently been developed based on double-transgenic Myc/Bcl-XL mice, the activation of MYC under the control of a light chain gene, or cloning of a spliced form of mouse XBP-1 downstream of the immunoglobulin VH promoter and enhancer elements. Although they recapitulate several characteristics of MM, these models are time-consuming and costly, perhaps explaining their limited use thus far. In Cinoxacin summary, the available MM models presented above can be technically challenging and require large investments. Thus, there is a need for an MM model where MM cells can be grown in vitro and when i.v. injected in a common laboratory inbred mouse strain, such as BALB/c, faithfully duplicate the 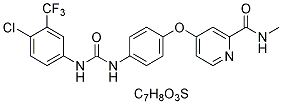 major characteristics of MM disease seen in patients. Plasmacytomas can be experimentally induced in certain strains of mice by i.p. injection of mineral oil, adjuvants and alkanes. Such mineral oil-induced plasmacytomas can be serially transplanted s.c. or i.p. and have been extensively used in tumor immunological studies. However, these plasmacytomas typically grow locally at the site of injection, and only infrequently metastasize to the bone marrow. Due to their local growth, it has been questioned if MOPC tumors represent good models for human MM that primarily affects bone marrow. We have previously described an in vivo-selected variant of MOPC315, MOPC315.4, which efficiently forms local tumors after s.c. injection. We here show that repeated i.v. injections of MOPC315.4 cells, followed by isolation of tumor cells from femurs between passages, enriches for a stable variant that can be grown in vitro, has tropism for bone marrow after i.v. injection, and causes osteolytic lesions. Spatiotemporal development of disease may be monitored by serial and noninvasive measurement of the bioluminescent signal of luciferase-labeled cells. Luciferase-labeled cells allow spatiotemporal resolution of bone disease development by repeated in vivo imaging. Injected mice develop osteolytic lesions, a hallmark of human MM. This novel mouse MM model could be useful for studies of bone marrow tropism, efficacy of drugs, mechanism of osteolysis, and immunotherapy.
major characteristics of MM disease seen in patients. Plasmacytomas can be experimentally induced in certain strains of mice by i.p. injection of mineral oil, adjuvants and alkanes. Such mineral oil-induced plasmacytomas can be serially transplanted s.c. or i.p. and have been extensively used in tumor immunological studies. However, these plasmacytomas typically grow locally at the site of injection, and only infrequently metastasize to the bone marrow. Due to their local growth, it has been questioned if MOPC tumors represent good models for human MM that primarily affects bone marrow. We have previously described an in vivo-selected variant of MOPC315, MOPC315.4, which efficiently forms local tumors after s.c. injection. We here show that repeated i.v. injections of MOPC315.4 cells, followed by isolation of tumor cells from femurs between passages, enriches for a stable variant that can be grown in vitro, has tropism for bone marrow after i.v. injection, and causes osteolytic lesions. Spatiotemporal development of disease may be monitored by serial and noninvasive measurement of the bioluminescent signal of luciferase-labeled cells. Luciferase-labeled cells allow spatiotemporal resolution of bone disease development by repeated in vivo imaging. Injected mice develop osteolytic lesions, a hallmark of human MM. This novel mouse MM model could be useful for studies of bone marrow tropism, efficacy of drugs, mechanism of osteolysis, and immunotherapy.
The cellular response to hypoxia is mainly control for a role of gutderived antigens in the onset of the NASH
Apoliprotein E is a ligand found in remnant lipoproteins that is recognized by various receptors in the liver. In humans, ApoE deficiency, or the presence of mutant forms of ApoE, results in type III hyperlipidemia characterized by the presence of elevated VLDL lipoproteins and early age onset of atherosclerosis. ApoE deficient mice are a widely used model of atherosclerosis, hyperlipidemia and steatosis. Thus, while ApoE2/2 mice develop a severe hyperlipidemia and atherosclerosis on a standard diet, they fail to develop liver inflammation, unless exposed to an additional hitting agent, making this setting a suitable model for testing the effects of therapeutic intervention on progression of lipid-related disorders in the liver and cardiovascular system. In the present study we have investigated the effects of VSL#3, a mixture of eight probiotic strains, in the progression of liver and vascular damage caused by challenging ApoE�C/�C with a low concentration of dextrane sulphate sodium, a well characterized intestinal barrier braking agent. The results of these studies demonstrate that a low grade inflammation increases intestinal permeability and leads to insulin resistance, transition from steatosis to NASH and exacerbated atherosclerosis and that all these disorders are efficiently prevented by a therapeutic intervention with a probiotic preparation. The study establishes that intervention on the intestinal microbiota is an effective therapeutic option in the treatment of systemic disorders. Because probiotic intervention resets immunoactivation and metabolism in multiple organs, we have then investigated whether it modulate the expression of nuclear receptors involved in reciprocal regulation of immune system and metabolism. Previous studies have established a role for nuclear receptors in mediating the effects of probiotics in rodent models of inflammation. Because an inverse regulation exists between several members of nuclear receptor superfamily and inflammation, we have assessed whether Lomitapide Mesylate products of probiotic metabolism might directly regulate the activity of these regulatory factors. The growing understanding of the functional role of human gut microbiota is showing that this enormous microbial population is instrumental in the control of host energy and lipid metabolism. Thus, while metagenomic studies are progressively deciphering the role of Tulathromycin B bacterial genes and proteins in the regulation of host’s metabolism, specific bacterial enterotypes have been associated to the development of human diseases such as diabetes and obesity. Despite the relation of the intestinal microbiota with the host is mutual, the mechanisms by which the intestinal immune system copes with the gut microbiota to contain local inflammation and prevent systemic dysregulation of immunity and metabolism are still poorly defined. In this report we have shown that low grade intestinal inflammation induced by administering ApoE2/2 mice with DSS results in a widespread inflammation whose signature markers were a systemic shift toward a Th1 phenotype along with a severe deterioration of the insulin signalling in the liver and adipose tissue. Because these changes were prevented by a probiotic intervention, these results highlight the central role of the intestinal microbiota in the pathogenesis of heretofore seemingly unrelated systemic inflammatory and metabolic disorders. Oxygen is essential for the survival of all eukaryotic cells, and metazoans are heavily dependent on this element to meet their large metabolic demands. At the cellular level, 90% of oxygen is consumed in oxidative phosphorylation. Consistent with a central role of oxygen in aerobic metabolism, all metazoan cells respond to  an imbalance between demand and supply of oxygen by activating a gene expression program aimed at restoring oxygen supply and reducing its consumption.
an imbalance between demand and supply of oxygen by activating a gene expression program aimed at restoring oxygen supply and reducing its consumption.
The PfRh ligands are located in the rhoptries of merozoites and specific mechanisms are in place in eukaryotic cells
Given the enormous implications in regulation of cellular function by the modulation of gene expression that regulate gene expression. Transcription regulatory machinenary in Folinic acid calcium salt pentahydrate eukaryotes involves specific transcription factors and transcription inhibitors; proteins that are required to turn on and turn off the expression of particular genes. These considerations point to a possible mechanism of how oxalate differentially regulates the gene expression of such a large number of genes. Given that oxalate is a metabolic end product in humans that cannot be further metabolized, such large scale changes in gene expression in renal epithelial cells in response to high oxalate levels points to an indirect mechanism of action, which may involve the interaction of oxalate with the cell membrane or in intracellular components. The primary site of oxalate action in cell remains unknown. Irrespective of primary site of action, one of the most common means by which cells sense changes is by activating the signal transduction pathways, especially the stress signal pathways. The stress associated signals are transduced through a series of proteins that are activated by phosphorylation/dephosphorylation steps and are finally turned into transcription factors, causing changes in gene expression. Though the present study design does not allow for the identification of activity changes due to phosphorylation, we identified changes in the gene expressions of upstream activators of several signaling pathways. Proteins like Ras, Fas and MKK are highly up-regulated as a result of oxalate exposure. These proteins are known to play important roles in JNK/SAPK signaling and p38 MAPK signaling. These results are in agreement with previous studies, by us and others, that identified an active role for Stress Activated Protein Kinases in oxalate renal cell interactions. We also identified changes in the expression of genes associated with retinoic Acid Receptor Signaling Pathway. Clearly additional studies are required to evaluate the functional consequence of these gene expression changes. In summary, our study is the first attempt at profiling the Genome-wide expression changes in human renal 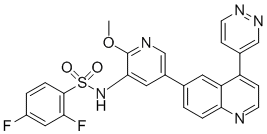 epithelial cells as a result of exposure to oxalate. Results from our study point to complex and intricate mechanisms, including differential gene expression, in renal epithelial cells in response to oxalate exposure. Clearly further studies are required to completely understand the implications of the plethora of changes in gene expression occurring as a result of oxalate exposure in renal epithelial cells. We must separate and characterize the genes that are derived from the by-stander effect and identify the genes whose altered expression is responsible for oxalate nephrotoxicity. Malaria due to Plasmodium falciparum remains a major global health burden and a leading cause of death worldwide among children under five. Benzethonium Chloride Increasing drug resistance, including emerging resistance to the artemisinin drugs, and the declining efficacy of vector control interventions in some populations make the development of effective malaria vaccines an urgent priority. During blood-stage infection, P. falciparum merozoites invade erythrocytes, mediated by the release of invasion ligands from apical organelles that interact with receptors on the erythrocyte surface. The repertoire of invasion ligands includes two major families, the P. falciparum reticulocyte-binding homologues, and erythrocyte binding antigens. The ability of P. falciparum to vary the expression and/or use of EBA and PfRh proteins enables the use of alternate invasion pathways, facilitating immune evasion that enables P. falciparum to cause repeated and chronic infections. Invasion pathways can be broadly classified into two main pathways, sialic acid dependent invasion and SA-independent invasion.
epithelial cells as a result of exposure to oxalate. Results from our study point to complex and intricate mechanisms, including differential gene expression, in renal epithelial cells in response to oxalate exposure. Clearly further studies are required to completely understand the implications of the plethora of changes in gene expression occurring as a result of oxalate exposure in renal epithelial cells. We must separate and characterize the genes that are derived from the by-stander effect and identify the genes whose altered expression is responsible for oxalate nephrotoxicity. Malaria due to Plasmodium falciparum remains a major global health burden and a leading cause of death worldwide among children under five. Benzethonium Chloride Increasing drug resistance, including emerging resistance to the artemisinin drugs, and the declining efficacy of vector control interventions in some populations make the development of effective malaria vaccines an urgent priority. During blood-stage infection, P. falciparum merozoites invade erythrocytes, mediated by the release of invasion ligands from apical organelles that interact with receptors on the erythrocyte surface. The repertoire of invasion ligands includes two major families, the P. falciparum reticulocyte-binding homologues, and erythrocyte binding antigens. The ability of P. falciparum to vary the expression and/or use of EBA and PfRh proteins enables the use of alternate invasion pathways, facilitating immune evasion that enables P. falciparum to cause repeated and chronic infections. Invasion pathways can be broadly classified into two main pathways, sialic acid dependent invasion and SA-independent invasion.
The viability of umbilical cord as a stem cell source is supported by the reports of several studies
Trp155 is not conserved in any if the bacterial genomes available at present, while the appearance of Phe at equivalent position to Phe150 is rare. Thermophilic enzymes frequently present increased number of clustered aromatic residues, which are often mutated to Leu in the mesophilic counterparts, as is the case in cvPAH. Surfaceexposed small aromatic clusters, often located close to the active sites, have been found to confer an entropic advantage over mesophilic analogues through generation of low-frequency motions. Based on the high sequence identity with the other PAH enzymes of known 3D-structure, Trp155 would be located at the start of the helix leading to the iron-coordinating residue Glu167 and might form a stabilizing aromatic cluster with Phe150 and/or Phe92. Furthermore, it is also well established that oligomerization is a strong stabilization mechanism and a large proportion of hyperthermophilic proteins have a higher oligomerization state than their mesophilic counterparts. In this context it is interesting that thermostable lpPAH appears to be dimeric, while other purified and characterized bacterial PAHs are monomeric. The clinical Butenafine hydrochloride symptoms and signs of ARF manifest as a rapid loss of the ability of the kidneys to excrete wastes, concentrate urine, and maintain fluid and electrolyte homeostasis. In pathophysiology, ARF may result from prolonged renal hypoperfusion and renal ischemia or nephrotoxic substances, and is associated with tubular cell death and shedding of cells into the tubular lumen, resulting in tubular blockage and further decreasing glomerular filtration. The overall mortality rate of patients with ARF is still high despite major advances in pharmacologic therapy, intensive care, and renal replacement therapy. Therefore, a more potent therapeutic intervention for ARF to reduce mortality is imperative. Our previous study showed that endogenous bone marrow cells could contribute to the renal tubular epithelial cell population and regeneration of the renal tubular epithelium by DNA synthesis after folic acid-induced acute kidney injury, although most of the renal tubular regeneration came from indigenous cells. These results have also been supported by another study. Recently, a stem cell-based treatment strategy has started to become a realistic option to replace or rebuild damaged organs and tissues. Stem cell therapy has been successfully applied using a variety of cell types, including mesenchymal stem cells, BM cells, and human umbilical cord blood cells, to rescue organ 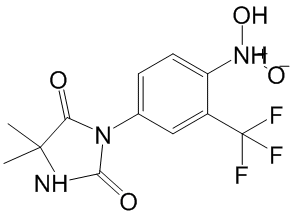 Albaspidin-AA damage in animal and human studies. On the basis of studies of allogeneic BM MSCs as a cell source for stem cell therapy for acute tubular necrosis, several studies have shown that sorted BM MSCs can rescue nonirradiated mice from acute renal tubular damage caused by toxins or ischemia; however, it is still debatable whether the beneficial effects of MSCs are primarily mediated via their differentiation into target cells, or by complex paracrine actions. BM MSCs are obtained from human bone marrow; however, aspiration of BM is an invasive procedure, and the numbers and differentiation capabilities of BM MSCs decline significantly with age. Fetal MSCs are derived from fetuses, a source associated with considerable ethical problems for human application, making these cells difficult to obtain. Compared with BM MSCs and fetal MSCs, human umbilical cord-derived mesenchymal stem cells can be separated from discarded umbilical cord, which causes no harm to the donor, and is not ethically problematic. Therefore, hUC-MSCs are a safe and accessible source for large quantities of stem cells in comparison to fetal MSCs and BM MSCs.
Albaspidin-AA damage in animal and human studies. On the basis of studies of allogeneic BM MSCs as a cell source for stem cell therapy for acute tubular necrosis, several studies have shown that sorted BM MSCs can rescue nonirradiated mice from acute renal tubular damage caused by toxins or ischemia; however, it is still debatable whether the beneficial effects of MSCs are primarily mediated via their differentiation into target cells, or by complex paracrine actions. BM MSCs are obtained from human bone marrow; however, aspiration of BM is an invasive procedure, and the numbers and differentiation capabilities of BM MSCs decline significantly with age. Fetal MSCs are derived from fetuses, a source associated with considerable ethical problems for human application, making these cells difficult to obtain. Compared with BM MSCs and fetal MSCs, human umbilical cord-derived mesenchymal stem cells can be separated from discarded umbilical cord, which causes no harm to the donor, and is not ethically problematic. Therefore, hUC-MSCs are a safe and accessible source for large quantities of stem cells in comparison to fetal MSCs and BM MSCs.