In addition protects the underlying tissues and thereby facilitating invasion. Patients suffering from COPD, for example, are frequently colonized by large numbers of Gram-negative or Grampositive bacteria, which produce and release large quantities of LPS, PG and LTA. Although the PAMP concentration required to elicit increased gliotoxin production in our experiments might be high as compared to typical systemic concentrations of bacteria infected patients, the local concentrations of PAMPs on the infected mucosal surfaces of the lung is likely to be much higher. In summary, we propose a theory that would explain how a bacterial infection possibly aggravates the mold co-infection rather than the traditional view that the mold infection clears a path for subsequent bacterial co-infections. In support of this, a recent study of aspergillosis patients showed that a systemic antibacterial treatment targeting the frequent co-infecting Pseudomonas aeruginosa, had a side effect of significantly diminishing the presence of Asperigillus fumigatus in the lungs. These results therefore advocate further investigation of the need to prioritize preventive suppression of pulmonary bacterial colonization in patients that have a high risk of contracting aspergillosis. Despite significant advances in anesthetic and surgical techniques, postoperative atrial fibrillation remains the most common complication after cardiac surgery. The incidence of POAF varies from 11% to 40%, depending on the definition and the method of monitoring. Although this arrhythmia is usually benign and self-limiting, it may result in hemodynamic Isoacteoside instability, a longer hospital stay, and increased health care costs. Given the clinical consequences a ributable to POAF, its prevention is of great importance. To date, many pharmacologic approaches have been a empted to prevent POAF, for example, b-blockers, amiodarone, and magnesium. Most reviews reflect a growing consensus in favor of the prophylactic administration of b-blockers for cardiac surgery patients. In addition, updated American College of Cardiology/American Heart Association 2006 guidelines recommend bblockers for the prevention of POAF. Despite the extensive studies, the exact pathophysiology of POAF 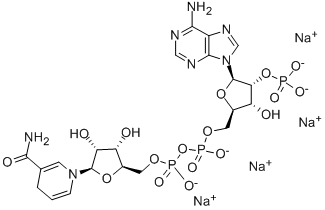 is for the moment far from being fully elucidated. A growing body of evidence suggests that markers of inflammation and oxidative injury are elevated in atrial fibrillation patients. Carvedilol, a non-selective badrenergic blocking agent approved for use in heart failure cases, has a number of ancillary activities including antiinflammatory and antioxidant properties. Moreover, unlike other beta-blockers, carvedilol antagonizes the rapiddepolarizing sodium channel, the human ether-a-go-go-related gene potassium channel, and the L-type calcium channel, which suggests a pharmacologic profile similar to amiodarone, a proven anti-arrhythmic agent for the prevention of POAF. Theoretically, this should reduce the incidence of arrhythmia, including POAF. All these properties of carvedilol have generated interest in its use as a prophylactic agent for POAF. Recently, several Coptisine-chloride relevant studies regarding prophylactic carvedilol in preventing POAF have been published. However, the role of carvedilol in preventing POAF remains unknown.
is for the moment far from being fully elucidated. A growing body of evidence suggests that markers of inflammation and oxidative injury are elevated in atrial fibrillation patients. Carvedilol, a non-selective badrenergic blocking agent approved for use in heart failure cases, has a number of ancillary activities including antiinflammatory and antioxidant properties. Moreover, unlike other beta-blockers, carvedilol antagonizes the rapiddepolarizing sodium channel, the human ether-a-go-go-related gene potassium channel, and the L-type calcium channel, which suggests a pharmacologic profile similar to amiodarone, a proven anti-arrhythmic agent for the prevention of POAF. Theoretically, this should reduce the incidence of arrhythmia, including POAF. All these properties of carvedilol have generated interest in its use as a prophylactic agent for POAF. Recently, several Coptisine-chloride relevant studies regarding prophylactic carvedilol in preventing POAF have been published. However, the role of carvedilol in preventing POAF remains unknown.
Monthly Archives: April 2019
DNA methylation is a common regulatory process which influences cell activities including transcription
Therefore, the overexpression of 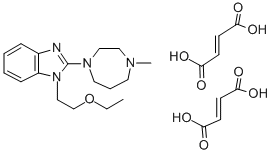 WRAP53 protein appears to play an important role in the progression of ESCC. In conclusion, our results have shown that the expression of WRAP53, the natural antisense transcript to p53, is significantly upregulated at the level of both mRNA and protein in ESCC tissues compared with non-neoplastic esophageal mucosa tissues. Overexpression of WRAP53 correlated with tumor infiltration depth, clinical stage, and lymph node metastasis. Therefore, WRAP53 may play a significant role in the development and progression of ESCC. Thus, WRAP53 could be a useful biomarker for ESCC and could represent a potential target for treatments of this disease. Glioblastoma is the most malignant primary brain tumor in adults with an overall survival rate of about 1.5 years even when treated with radical regimens including surgical resection, and radiotherapy with concomitant and/or adjuvant temozolomide chemotherapy. Although the exact mechanism of GBM development and progression is still unknown, certain molecular biomarkers are related to tumorigenesis and progression of GBM at the genetic, epigenetic, and transcriptional levels. However, markers for GBM that have prognostic value in signaling transduction pathways have not been fully elucidated yet.The Janus kinase/signal transducer and activator of transcription 3 signaling pathways transmits extracellular signals into the nucleus where it regulates DNA transcription and activity in the cell. The suppressor of cytokine signaling 3 is an endogenous inhibitor of the JAK/STAT3 signaling pathway, modulating cell activities via suppressing transcription. Recently, some studies have reported that SOCS3 functions as a tumor suppressor in multiple tumor types, including GBM. DNA methylation is a precisely regulated process in normal cells that becomes drastically modified in cancer cells. Atractylenolide-III Hypomethylation of oncogene promoters and hypermethylation of tumor suppressor gene promoters are pivotal alterations in cancer development. Moreover, DNA methylation is typically a stable and inheritable epigenetic pattern that can persist for several cell generations, which potentially broadens its clinical practical applicability. Hypermethylation of oncogenic genes is a favorable indictor for GBM patients. A variety of studies have reported that hypermethylation of the SOCS3 promoter predicts poor prognosis in certain cancers, including GBM. However, in our study, hypermethylation of the SOCS3 promoter was Benzoylpaeoniflorin associated with better outcomes for GBM patients. In addition, we found that hypermethylation of the SOCS3 promoter in GBM was tightly associated with the G-CIMP-positive GBM patients. Glioblastoma is the most malignant primary brain tumor in adults, with insidious development, rapid progression and poor outcomes. Alterations in cell signaling pathways may be associated with the development and progression of GBM. Some prognostic bio-markers involved in signaling pathways have been identified. Hypermethylation of the SOCS3 promoter has been associated with a poor outcome for GBM. From our present research, we draw an opposite conclusion to previous studies and show that hypermethylation of the SOCS3 promoter predicts an improved prognosis for GBM patients.
WRAP53 protein appears to play an important role in the progression of ESCC. In conclusion, our results have shown that the expression of WRAP53, the natural antisense transcript to p53, is significantly upregulated at the level of both mRNA and protein in ESCC tissues compared with non-neoplastic esophageal mucosa tissues. Overexpression of WRAP53 correlated with tumor infiltration depth, clinical stage, and lymph node metastasis. Therefore, WRAP53 may play a significant role in the development and progression of ESCC. Thus, WRAP53 could be a useful biomarker for ESCC and could represent a potential target for treatments of this disease. Glioblastoma is the most malignant primary brain tumor in adults with an overall survival rate of about 1.5 years even when treated with radical regimens including surgical resection, and radiotherapy with concomitant and/or adjuvant temozolomide chemotherapy. Although the exact mechanism of GBM development and progression is still unknown, certain molecular biomarkers are related to tumorigenesis and progression of GBM at the genetic, epigenetic, and transcriptional levels. However, markers for GBM that have prognostic value in signaling transduction pathways have not been fully elucidated yet.The Janus kinase/signal transducer and activator of transcription 3 signaling pathways transmits extracellular signals into the nucleus where it regulates DNA transcription and activity in the cell. The suppressor of cytokine signaling 3 is an endogenous inhibitor of the JAK/STAT3 signaling pathway, modulating cell activities via suppressing transcription. Recently, some studies have reported that SOCS3 functions as a tumor suppressor in multiple tumor types, including GBM. DNA methylation is a precisely regulated process in normal cells that becomes drastically modified in cancer cells. Atractylenolide-III Hypomethylation of oncogene promoters and hypermethylation of tumor suppressor gene promoters are pivotal alterations in cancer development. Moreover, DNA methylation is typically a stable and inheritable epigenetic pattern that can persist for several cell generations, which potentially broadens its clinical practical applicability. Hypermethylation of oncogenic genes is a favorable indictor for GBM patients. A variety of studies have reported that hypermethylation of the SOCS3 promoter predicts poor prognosis in certain cancers, including GBM. However, in our study, hypermethylation of the SOCS3 promoter was Benzoylpaeoniflorin associated with better outcomes for GBM patients. In addition, we found that hypermethylation of the SOCS3 promoter in GBM was tightly associated with the G-CIMP-positive GBM patients. Glioblastoma is the most malignant primary brain tumor in adults, with insidious development, rapid progression and poor outcomes. Alterations in cell signaling pathways may be associated with the development and progression of GBM. Some prognostic bio-markers involved in signaling pathways have been identified. Hypermethylation of the SOCS3 promoter has been associated with a poor outcome for GBM. From our present research, we draw an opposite conclusion to previous studies and show that hypermethylation of the SOCS3 promoter predicts an improved prognosis for GBM patients.
CCNA2 is down-regulated in MCF-7 tumor treated with tamoxifen compared with control in vivo
However, tamoxifen has no significant influence on CCNA2 expression in tamoxifen resistant subclones, which indicates CCNA2’s potential role in tamoxifen resistance. Nevertheless, whether CCNA2 is a driver gene in tamoxifen resistance still requires experimental validation. Provided the high expression of CCNA2 is involved in the 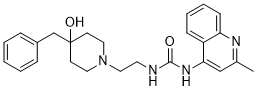 development of tamoxifen resistance, how to manage cancer patients with CCNA2 overexpression remains a great challenge. Here we show several drugs that could influence the expression of CCNA2. For instance, Doxorubicin could decrease the expression of CCNA2, while Oxaliplatin could increase the expression of CCNA2. However, whether ER+ breast cancer patient with CCNA2 overexpression could benefit from the repression of CCNA2, or in other words, whether CCNA2 is a promising target for preventing or could reverse tamoxifen resistance still needs more experimental support. Although much information about ER and cancer has been provided in the past three decades since the arrival of tamoxifen in the clinic, a lot more needs to be elucidated for favorable therapeutic outcomes. More concrete research outcomes will warrant the translational research that may lead to more efficient and safer treatment for breast cancer patients as well as women at high risk of advanced breast cancer. Taken together, this study indicates that CCNA2 expression may help monitoring tamoxifen efficacy. In addition, it suggests the relevance of CCNA2 in the development of tamoxifen resistance. Furthermore, it could provide guidance personalized therapies. Nevertheless, multi-center randomized controlled clinical trials and in vivo/in vitro experiments are still needed before its application in clinical settings. Natural antisense transcripts, also called antisense RNAs, are RNAs that contain sequences that are complementary to other endogenous transcripts. Antisense RNAs may also encode proteins or may exist only as non-protein-coding transcripts. In recent years, investigations into NAT functions have indicated that NATs play key roles in carcinogenesis and the development of cancers. The tumor suppressor gene TP53 is the most frequently mutated gene in human cancers. P53 is a pivotal tumor suppressor that induces apoptosis, cell-cycle arrest, and senescence in response to stress signals such as DNA damage, hypoxia, or activated oncogenes. A natural antisense transcript to p53 has recently been identified; WRAP53 gives rise to p53 antisense transcripts that regulate p53 mRNA expression and are required for p53 activity upon DNA damage. WRAP53 transcripts may also be translated into WRAP53 protein, supporting the proliferation of progenitor cells and tumor cells by binding to telomerase to add telomere repeats to Danshensu chromosome ends. Esophageal cancer is one of the most common malignant tumors, resulting in poor prognosis worldwide. ESCC is the most frequent histological EC subtype, accounts for more than 90% of ECs, and results in clinical outcomes with high mortality rates in China. Esophageal carcinogenesis involves multiple cellular alterations, including Echinacoside aberrant cell cycle control, DNA repair, cellular enzymes, and growth factor and nuclear receptors.
development of tamoxifen resistance, how to manage cancer patients with CCNA2 overexpression remains a great challenge. Here we show several drugs that could influence the expression of CCNA2. For instance, Doxorubicin could decrease the expression of CCNA2, while Oxaliplatin could increase the expression of CCNA2. However, whether ER+ breast cancer patient with CCNA2 overexpression could benefit from the repression of CCNA2, or in other words, whether CCNA2 is a promising target for preventing or could reverse tamoxifen resistance still needs more experimental support. Although much information about ER and cancer has been provided in the past three decades since the arrival of tamoxifen in the clinic, a lot more needs to be elucidated for favorable therapeutic outcomes. More concrete research outcomes will warrant the translational research that may lead to more efficient and safer treatment for breast cancer patients as well as women at high risk of advanced breast cancer. Taken together, this study indicates that CCNA2 expression may help monitoring tamoxifen efficacy. In addition, it suggests the relevance of CCNA2 in the development of tamoxifen resistance. Furthermore, it could provide guidance personalized therapies. Nevertheless, multi-center randomized controlled clinical trials and in vivo/in vitro experiments are still needed before its application in clinical settings. Natural antisense transcripts, also called antisense RNAs, are RNAs that contain sequences that are complementary to other endogenous transcripts. Antisense RNAs may also encode proteins or may exist only as non-protein-coding transcripts. In recent years, investigations into NAT functions have indicated that NATs play key roles in carcinogenesis and the development of cancers. The tumor suppressor gene TP53 is the most frequently mutated gene in human cancers. P53 is a pivotal tumor suppressor that induces apoptosis, cell-cycle arrest, and senescence in response to stress signals such as DNA damage, hypoxia, or activated oncogenes. A natural antisense transcript to p53 has recently been identified; WRAP53 gives rise to p53 antisense transcripts that regulate p53 mRNA expression and are required for p53 activity upon DNA damage. WRAP53 transcripts may also be translated into WRAP53 protein, supporting the proliferation of progenitor cells and tumor cells by binding to telomerase to add telomere repeats to Danshensu chromosome ends. Esophageal cancer is one of the most common malignant tumors, resulting in poor prognosis worldwide. ESCC is the most frequent histological EC subtype, accounts for more than 90% of ECs, and results in clinical outcomes with high mortality rates in China. Esophageal carcinogenesis involves multiple cellular alterations, including Echinacoside aberrant cell cycle control, DNA repair, cellular enzymes, and growth factor and nuclear receptors.
This study aimed to determine if the expression of Mas receptor is altered in the kidneys of CKD rats
Therefore, the elucidation of how various MAPs exert 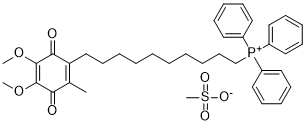 their effects on microtubules may have a profound impact on the diagnosis and therapy of many human diseases. Mdp3 has Epimedoside-A recently been characterized as a novel member of the MAP family. Intriguingly, Mdp3 interacts with microtubules/ tubulin and stabilizes microtubules through its amino-terminal region instead of its carboxyl-terminal MAP7 domain, indicating the structural diversity of the MAP7 domains present in different proteins. It would be interesting to investigate in the future whether the two closely related proteins, Mdp1 and Mdp2, interact with microtubules/tubulin and promote microtubule stability, and if so, whether these actions are mediated by their MAP7 domains. Further studies are also warranted to study the expression patterns of Mdp3 in different cell types and to analyze whether the expression or posttranslational modification of Mdp3 is altered in various human diseases. Stable microtubules are known to possess a high level of tubulin acetylation, although the causal relationship between tubulin acetylation and microtubule stability remains to be investigated. The acetyltransferases Elongator and MEC-17 have been reported to catalyze the tubulin acetylation process, and conversely the deacetylases HDAC6 and sirtuin 2 are able to remove the acetyl group from tubulin. The present study reveals that the amino-terminal region of Mdp3 mediates its interaction with HDAC6 in the cytoplasm, in addition to mediating Mdp3 binding to microtubules/tubulin. Interestingly, Mdp3 suppresses the activity of HDAC6, leading to enhanced tubulin acetylation. This finding suggests that Mdp3 might regulate microtubule stability by acting on HDAC6-mediated tubulin deacetylation, in addition to its direct association with microtubules/tubulin. HDAC6, as a unique member of the HDAC family with a predominant localization in the cytoplasm, has been implicated in numerous cellular processes such as cell motility, cell-cell interaction, and transcriptional regulation. HDAC6 carries out the above Gentiopicrin functions primarily through its deacetylation of substrate proteins such as a-tubulin, cortactin, and Hsp90. In addition, HDAC6 could interact with polyubiquitinated misfolded proteins and the dynein motor, facilitating the transport of misfolded proteins to the aggresome. Over the past decade, HDAC6 has emerged as a promising target for drug development due to its involvement in oncogenic cell transformation and neurodegeneration. In this scenario, our finding that Mdp3 is an endogenous inhibitor of HDAC6 may have important implications in health and diseases. The ACE2/Ang-/Mas axis is protective in renal disease, proven by the transgenic and knockout mouse models. However, the expression of ACE2 in diseased kidneys in previous studies is divergent. In addition, there are controversial data on the renoprotective effect of Ang-, including stimulation of inflammation by Ang-. Little is known about Mas receptor expression in CKD. Indoxyl sulfate, one of proteinbound uremic toxins, elicits a variety of cytotoxic effects, and exacerbates CKD. The role of IS on the Mas receptor has not been elucidated.
their effects on microtubules may have a profound impact on the diagnosis and therapy of many human diseases. Mdp3 has Epimedoside-A recently been characterized as a novel member of the MAP family. Intriguingly, Mdp3 interacts with microtubules/ tubulin and stabilizes microtubules through its amino-terminal region instead of its carboxyl-terminal MAP7 domain, indicating the structural diversity of the MAP7 domains present in different proteins. It would be interesting to investigate in the future whether the two closely related proteins, Mdp1 and Mdp2, interact with microtubules/tubulin and promote microtubule stability, and if so, whether these actions are mediated by their MAP7 domains. Further studies are also warranted to study the expression patterns of Mdp3 in different cell types and to analyze whether the expression or posttranslational modification of Mdp3 is altered in various human diseases. Stable microtubules are known to possess a high level of tubulin acetylation, although the causal relationship between tubulin acetylation and microtubule stability remains to be investigated. The acetyltransferases Elongator and MEC-17 have been reported to catalyze the tubulin acetylation process, and conversely the deacetylases HDAC6 and sirtuin 2 are able to remove the acetyl group from tubulin. The present study reveals that the amino-terminal region of Mdp3 mediates its interaction with HDAC6 in the cytoplasm, in addition to mediating Mdp3 binding to microtubules/tubulin. Interestingly, Mdp3 suppresses the activity of HDAC6, leading to enhanced tubulin acetylation. This finding suggests that Mdp3 might regulate microtubule stability by acting on HDAC6-mediated tubulin deacetylation, in addition to its direct association with microtubules/tubulin. HDAC6, as a unique member of the HDAC family with a predominant localization in the cytoplasm, has been implicated in numerous cellular processes such as cell motility, cell-cell interaction, and transcriptional regulation. HDAC6 carries out the above Gentiopicrin functions primarily through its deacetylation of substrate proteins such as a-tubulin, cortactin, and Hsp90. In addition, HDAC6 could interact with polyubiquitinated misfolded proteins and the dynein motor, facilitating the transport of misfolded proteins to the aggresome. Over the past decade, HDAC6 has emerged as a promising target for drug development due to its involvement in oncogenic cell transformation and neurodegeneration. In this scenario, our finding that Mdp3 is an endogenous inhibitor of HDAC6 may have important implications in health and diseases. The ACE2/Ang-/Mas axis is protective in renal disease, proven by the transgenic and knockout mouse models. However, the expression of ACE2 in diseased kidneys in previous studies is divergent. In addition, there are controversial data on the renoprotective effect of Ang-, including stimulation of inflammation by Ang-. Little is known about Mas receptor expression in CKD. Indoxyl sulfate, one of proteinbound uremic toxins, elicits a variety of cytotoxic effects, and exacerbates CKD. The role of IS on the Mas receptor has not been elucidated.
As highly connected local interaction hubs mediators of allosteric communication in the ternary complex
In addition, the average centrality of the Rar1-CHORD2 residues is higher than in the other domains, i.e. the key mediator residues are surrounded by “supporting cast” residues that have sufficient communication capacity to rapidly disseminate the information signal from central mediators across the network. Hence, the Rar1-CHORD2 binding could regulate the long-range interactions and promote stabilization of the ternary complex. The network analysis could thus clarify the elusive role of Rar1 as a key component of the ternary assembly and stability enhancer of the Hsp90-cochaperone interactions. Overall, our results support the mechanism in which a selected group of critical Rar1CHORD2 residues, particularly Rar1-W217 as a potential principal contributor, may be critical for mediating long-range interactions and modulation of the Hsp90-ATPase activity. Our results indicated that the Rar1-CHORD2 binding may result in a more assortative interaction network that is better integrated through preferential association of global mediating residues with many locally connected hubs. The centrality analysis of the Hsp90-Cdc37 Praeruptorin-B complex similarly revealed that key mediating residues and functional hot spots are aligned with the peaks in the closeness and betweenness profiles. The characteristic peaks were conserved in both distributions and corresponded in the Hsp90NTD to the residues E47 and Q133. Both residues form stabilizing polar interactions with R167 in Cdc37 and these interactions are implicated as a major contributing factor in the mechanism of Cdc37-mediated inhibition of the ATPase activity. The centrality analysis recovered major functional sites E47, Q133, and F134 as key mediating residues of allosteric communications in the Hsp90-Cdc37 complex. Interestingly, E47A mutation reduced Hsp90-Cdc37 binding by 50%, while Q133A mutation could decrease  the Hsp90-Cdc37 interactions by 85%. While our analysis 14alpha-hydroxy-Sprengerinin-C robustly selected the Hsp90 binding hot spots, the simplicity of the centrality metric somewhat underestimated the relative contribution of Q133 to the Hsp90-Cdc37 binding that is more significant that was predicted. In the Cdc37MD, the centrality peaks corresponded to R167 and Q208 residues that were also among highly connected interfacial hubs. Overall, the centrality analysis suggested that the group of strongly interacting residues Hsp90-E47, Hsp90-Q133, Cdc37-R167, and Cdc37-Q208 could collectively form a major gateway for allosteric communications in the Hsp90-Cdc37 complex. These global mediating residues are also highly connected local hubs and major contributors of the structurally stable communities. The prominent role of these residues in the Hsp90-Cdc37 binding was demonstrated via a comprehensive biochemical analysis in which mutations of Hsp90-Q133, Cdc37-R167 and Cdc37-Q208 essentially abolished the complex formation, causing 85%�C90% reduction in the Hsp90-Cdc37 interactions as measured in cell-based assays. These key functional sites were consistently recovered as largest peaks in both closeness and betweenness metrics. Mutations of key mediating nodes in the Hsp90-Cdc37 complex would cause a simultaneous disruption of multiple interactions and disrupt the integrity of the allosteric network, thus leading to a dramatic loss of the chaperone activity. At the same time, the centrality analysis unveiled a few smaller noticeable peaks corresponding to the Hsp90-NTD residues Y61, L91 W162 and Cdc37-F238. These residues are not located at the interdomain interface and reside within their respective domains, contributing to the structural integrity of the interacting modules.
the Hsp90-Cdc37 interactions by 85%. While our analysis 14alpha-hydroxy-Sprengerinin-C robustly selected the Hsp90 binding hot spots, the simplicity of the centrality metric somewhat underestimated the relative contribution of Q133 to the Hsp90-Cdc37 binding that is more significant that was predicted. In the Cdc37MD, the centrality peaks corresponded to R167 and Q208 residues that were also among highly connected interfacial hubs. Overall, the centrality analysis suggested that the group of strongly interacting residues Hsp90-E47, Hsp90-Q133, Cdc37-R167, and Cdc37-Q208 could collectively form a major gateway for allosteric communications in the Hsp90-Cdc37 complex. These global mediating residues are also highly connected local hubs and major contributors of the structurally stable communities. The prominent role of these residues in the Hsp90-Cdc37 binding was demonstrated via a comprehensive biochemical analysis in which mutations of Hsp90-Q133, Cdc37-R167 and Cdc37-Q208 essentially abolished the complex formation, causing 85%�C90% reduction in the Hsp90-Cdc37 interactions as measured in cell-based assays. These key functional sites were consistently recovered as largest peaks in both closeness and betweenness metrics. Mutations of key mediating nodes in the Hsp90-Cdc37 complex would cause a simultaneous disruption of multiple interactions and disrupt the integrity of the allosteric network, thus leading to a dramatic loss of the chaperone activity. At the same time, the centrality analysis unveiled a few smaller noticeable peaks corresponding to the Hsp90-NTD residues Y61, L91 W162 and Cdc37-F238. These residues are not located at the interdomain interface and reside within their respective domains, contributing to the structural integrity of the interacting modules.