Sortilin, the polarity protein Crumbs and Wls. Drosophila and mammalian tissue culture cells. Retromer mediated recycling of Wls is required for Wnt signaling in invertebrate model systems, but the in vivo role of this retrieval pathway has not been tested in mammalian Wnt signaling. We generated a floxed allele of Vps35 to conditionally interfere with retromer function in the murine intestinal epithelium. We investigated the effect of Vps35 deletion in vivo, and in a recently established intestinal organoid culture system. We show that Vps35 is required to maintain Wls protein levels in intestinal cells, but growth of intestinal organoids was only mildly affected. This suggests that retromer mediated recycling of Wls is dispensable in the mammalian intestinal epithelium in AbMole Benzyl alcohol steady state conditions. Retromer dependent recycling of Wls is essential for efficient Wnt secretion in C. AbMole Niflumic acid elegans, Drosophila and mammalian tissue culture cells, but the in vivo role of Wls recycling in mammalian Wnt signaling has not been tested. In the murine intestine, Wnt signaling is required for proliferation and stem cell maintenance. We therefore investigated whether the retromer complex is required for stem cell maintenance and tissue homeostasis in the intestine. This proliferation defect cannot be completely rescued by supplementation of Wnt3a in the medium, indicating that Vps35 controls organoid growth through both Wnt dependent and Wnt independent effects. Why does loss of Vps35 and the resulting reduction in Wls protein levels cause only a subtle proliferation defect in the mouse intestine? In C. elegans, loss of the retromer complex mainly 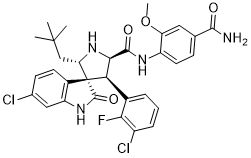 affects Wnt signaling processes that act over a relatively long distance, such as neuroblast migration and the establishment of neuronal polarity. These Wnt signaling processes require the formation of long-range Wnt concentration gradients and are therefore dependent on efficient Wnt secretion. In contrast, loss of retromer function does not significantly affect Wnt signaling processes that take place between neighboring cells. Also in the Drosophila wing imaginal disc, loss of retromer mainly affects the expression of high threshold Wnt target genes, while low threshold target genes such as Distalless are normally expressed. Taken together, these studies show that loss of retromer reduces, but not eliminates Wnt secretion in worms and flies. Stem cells in intestinal organoids require stimulation by Wnt proteins that are secreted from neighboring Paneth cells. In analogy with C. elegans, this short range Wnt signaling may be less sensitive to loss of Vps35. In addition, Wnt signaling in intestinal stem cells is amplified by Rspondin, a ligand that acts through the Lgr5 receptor.
affects Wnt signaling processes that act over a relatively long distance, such as neuroblast migration and the establishment of neuronal polarity. These Wnt signaling processes require the formation of long-range Wnt concentration gradients and are therefore dependent on efficient Wnt secretion. In contrast, loss of retromer function does not significantly affect Wnt signaling processes that take place between neighboring cells. Also in the Drosophila wing imaginal disc, loss of retromer mainly affects the expression of high threshold Wnt target genes, while low threshold target genes such as Distalless are normally expressed. Taken together, these studies show that loss of retromer reduces, but not eliminates Wnt secretion in worms and flies. Stem cells in intestinal organoids require stimulation by Wnt proteins that are secreted from neighboring Paneth cells. In analogy with C. elegans, this short range Wnt signaling may be less sensitive to loss of Vps35. In addition, Wnt signaling in intestinal stem cells is amplified by Rspondin, a ligand that acts through the Lgr5 receptor.
Vps35 is the central cargo-binding subunit of the retromer complex strongly reduces Wnt secretion in Celegans
Leave a reply