After AbMole beta-Eudesmol self-crossing, a portion of the seeds, specifically, 25% of the seeds, will lose the transgene. Several self-crosses and generations are needed to obtain transgene-homozygous plants and seeds. This can be time and labor consuming. Also, the amount of seeds developed from a plant is often limited. A large number of plants need to be grown to obtain sufficient seeds for research, especially commercial uses. This needs large spaces and land, long periods of time and extensive labor input. In addition, seed development in some plant species is naturally impaired due to various reasons and thus transgenes may not be passed to the next generation and transgenic 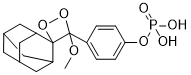 materials can be lost. Moreover, perennial plants and woody plants need a much longer time to produce seeds. As such, the breeding process to pass transgenes to the next generation in these types of plants can be very slow. Plants have unique characteristics that allow various cells, after certain induction, to reprogram and develop into somatic embryos. Somatic embryos have the same morphology and structure as zygotic embryos and can germinate and develop into full and fertile plants. Somatic embryogenesis has been developed in a large number of plant species and the system has been used widely for producing transgenic plants for molecular biology and functional genomics research and in biotechnology for plant trait improvement. Somatic embryos, after certain treatments such as abscisic acid, sucrose and heat shock, can acquire tolerance to water loss. They can be dried to contain less than 15% water, similar to the water content in true seeds, and still remain viable under ambient environment. After rehydration, the somatic embryos can germinate and develop into full plants. Dried somatic embryos can be intact as they are produced or encapsulated and they are collectively called artificial seeds or synthetic seed. Artificial seeds can be stored for long periods of time and still possess propagation ability. These embryos can be handled or shipped as true seeds. Artificial seeds indeed are a true analog of conventional seeds and can be used for germplasm and genetic material preservation. Artificial seed technology and artificial seedrelated technology have been reported in various plant species. Induction of somatic embryos from transgenic plants and the use of artificial seeds may provide a new system for transgene preservation. Here, we report stable transgene preservation and faithful expression of a transgene in plants developed from dried somatic embryos in alfalfa. The new system can be used to preserve transgenic materials for research use and preserve transgenic germplasm for applications in different plant species. Fluorometric analysis was conducted to measure GUS enzyme activity in leaf tissues as described by Jefferson et al.. Protein content in the extract was determined spectrophotometrically according to Bradford using a commercially available Bradford Reagent dye. Measurements of the enzyme activity were repeated 2�C4 times after incubation lasting from 15 min to 24 hours depending on the levels of sample fluorescence. GUS activity was expressed as pM 4-MU per mg protein per minute. Somatic embryos were induced from different and independent transgenic plants using petioles as explants. The procedure was the same as the transformation method but without Agrobacterium infection. Cotyledonary-staged somatic embryos of different transgenic alfalfa lines were randomly divided into two groups. One group was used for desiccation treatment and the other was used as controls. Transgenic alfalfa plants were obtained via Agrobacteriummediated transformation using the method well established in our laboratory. Plant transformation was confirmed by PCR using uid gene primers, histochemical analysis and Southern blot analysis.
materials can be lost. Moreover, perennial plants and woody plants need a much longer time to produce seeds. As such, the breeding process to pass transgenes to the next generation in these types of plants can be very slow. Plants have unique characteristics that allow various cells, after certain induction, to reprogram and develop into somatic embryos. Somatic embryos have the same morphology and structure as zygotic embryos and can germinate and develop into full and fertile plants. Somatic embryogenesis has been developed in a large number of plant species and the system has been used widely for producing transgenic plants for molecular biology and functional genomics research and in biotechnology for plant trait improvement. Somatic embryos, after certain treatments such as abscisic acid, sucrose and heat shock, can acquire tolerance to water loss. They can be dried to contain less than 15% water, similar to the water content in true seeds, and still remain viable under ambient environment. After rehydration, the somatic embryos can germinate and develop into full plants. Dried somatic embryos can be intact as they are produced or encapsulated and they are collectively called artificial seeds or synthetic seed. Artificial seeds can be stored for long periods of time and still possess propagation ability. These embryos can be handled or shipped as true seeds. Artificial seeds indeed are a true analog of conventional seeds and can be used for germplasm and genetic material preservation. Artificial seed technology and artificial seedrelated technology have been reported in various plant species. Induction of somatic embryos from transgenic plants and the use of artificial seeds may provide a new system for transgene preservation. Here, we report stable transgene preservation and faithful expression of a transgene in plants developed from dried somatic embryos in alfalfa. The new system can be used to preserve transgenic materials for research use and preserve transgenic germplasm for applications in different plant species. Fluorometric analysis was conducted to measure GUS enzyme activity in leaf tissues as described by Jefferson et al.. Protein content in the extract was determined spectrophotometrically according to Bradford using a commercially available Bradford Reagent dye. Measurements of the enzyme activity were repeated 2�C4 times after incubation lasting from 15 min to 24 hours depending on the levels of sample fluorescence. GUS activity was expressed as pM 4-MU per mg protein per minute. Somatic embryos were induced from different and independent transgenic plants using petioles as explants. The procedure was the same as the transformation method but without Agrobacterium infection. Cotyledonary-staged somatic embryos of different transgenic alfalfa lines were randomly divided into two groups. One group was used for desiccation treatment and the other was used as controls. Transgenic alfalfa plants were obtained via Agrobacteriummediated transformation using the method well established in our laboratory. Plant transformation was confirmed by PCR using uid gene primers, histochemical analysis and Southern blot analysis.